ALK Testing in Europe
By Enriqueta Felip, MD, PhD
Posted: February 2018
ALK-directed TKIs are effective first- and second-line therapies in patients with ALK rearrangements. Testing of all patients with...
Adjuvant Gefitinib Extended Disease-Free Survival in Patients With Stage II/IIIA Non-Small Cell Lung Cancer...
By Cynthia L. Kryder, MS, CCC-Sp
Editor Note: IASLC Lung Cancer News is pleased to provide the following overview, which is followed by expert commentary...
Osimertinib: A New Option in Non-Small Cell Lung Cancer – Q&A with David R....
By Erik J. MacLaren, PhD
Osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), received FDA approval in November 2015 for use...
Alectinib and ALK-Positive Non-Small Cell Lung Cancer – Q&A with Alice T. Shaw, MD,...
By Erik J. MacLaren, PhD
Alectinib is a second-generation anaplastic lymphoma kinase (ALK) inhibitor that was recently approved in the US as a second- line...
Benefit of EGFR Mutation Analysis from Plasma Cell-Free DNA for Treatment of Advanced Non-Small...
By Tony S. K. Mok, BMSc, MD, FRCPC
Patients must first be identified as having an epidermal growth factor receptor (EGFR) mutation before they can...
The Lung Master Protocol (Lung-MAP)
By Vassiliki A. Papadimitrakopoulou, MD
Lung-MAP (S1400), is a unique master multi-study protocol that incorporates genomic testing of tumors through a next-generation sequencing (NGS) platform...
Q & A with Yi-Long Wu, MD
What is the current standard of care in the adjuvant setting post-resection of high-risk NSCLC in your practice or nationally?
Posted: October 2017
Current Chinese Lung...
Clinical Labeling in Medicinal Products: An Interview With Dr. David Planchard About the Effects...
Posted: June 2017
By David Planchard, MD, PhD
What was the rationale for conducting clinical research in BRAF mutationpositive NSCLC?
Recently, progress has been made in characterization...
ADJUVANT Trial of Gefitinib Versus Chemotherapy in Resected EGFR-Mutations Non-Small Cell Lung Cancer
By Heather Wakelee, MD
Posted: October 2017
For most of the world, four cycles of cisplatin-based adjuvant chemotherapy is the standard of care for patients with...
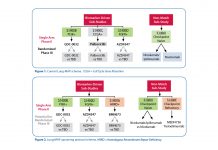
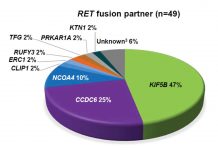
Uncommon Oncogenic Drivers in NSCLC
By Karen L. Reckamp, MD, MS
Posted: March 1, 2019
Identification of genetic alterations in NSCLC has transformed diagnosis and treatment for patients with advanced disease....