By John Armstrong, MD, FRCPI, DABR, FFRRCSI
Posted: December 2018
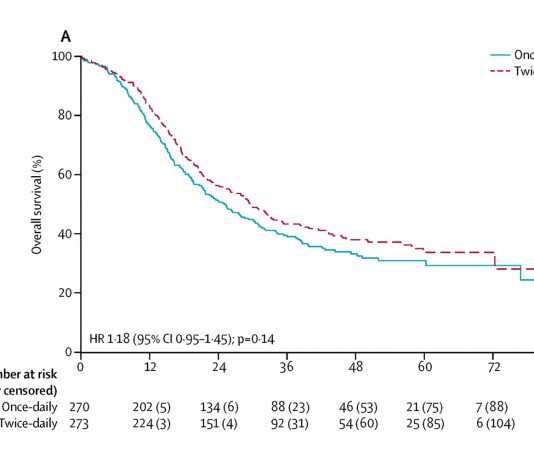
RTOG 0214, a phase III randomized trial, is the biggest trial to date testing prophylactic cranial irradiation (PCI) in locally advanced NSCLC.1 The trial, presented at the IASLC World Conference on Lung Cancer this past September, evaluated patients with stage III NSCLC whose disease had not progressed after treatment with surgery and/or radiation therapy with or without chemotherapy. PCI failed to improve survival, which could have been due to a number of factors, despite a reduction in brain metastases for patients who received PCI.

Study Details
Participants were stratified by stage (IIIA vs. IIIB), histology (nonsquamous vs. squamous), and therapy (surgery vs. none) and were randomly assigned to PCI or observation. The primary endpoint of the study was overall survival (OS). Of the 356 patients entered, 340 were eligible for analysis. The median follow-up time was 2.1 years for all patients, and 9.2 years for living patients.
The trial failed to meet accrual targets and was underpowered to detect a significant survival difference. PCI had no effect on OS. PCI did, however, significantly reduce the risk of brain metastases; patients in the observation arm were 2.33 times more likely to develop brain metastases than those in the PCI arm (p = 0.004). Nevertheless, there was a relative lack of efficacy in reducing brain metastases. The 10-year risk was 28.3% without PCI versus 16.7% with PCI. Thus, PCI yielded an absolute risk reduction of 11.6%. This means that PCI benefits just one in 8.6 patients. In contrast, in limited-stage small cell lung cancer (where PCI improves OS), PCI reduced the risk of brain metastases from 58% to 33%. For SCLC, PCI benefits one in four patients.2
Inadequate Effect Within the Brain
Completion of imaging prior to random assignment is important. The trial mandated CT scans or brain MRIs with or without contrast. However, CT imaging can miss a significant number of brain metastases detectable by MRI. The trial MRIs were done with and without contrast but were not necessarily volumetric studies. Therefore, an unknown number of patients on both arms of the trial probably had small, undetected brain metastases at enrolment.
In this group of patients with NSCLC, the risk of developing brain metastases was 28.3% without PCI versus 58% in the SCLC metanalysis.2 The biologic basis for PCI is that the brain represents a sanctuary site (perhaps because of the blood–brain barrier), where cancer cells survive the effects of chemotherapy. However, the relatively low baseline risk in this series suggests that the brain is not a sanctuary site and that failure in the brain is a manifestation of general failure of systemic control.
The reduction of risk from 28.3% without PCI to 16.6% denotes a 40% control rate. Therefore, PCI failed to eliminate 60% of occult or microscopic brain metastases. The dose used was 30 Gy in 15 fractions, which is probably a very low biologic dose. When biologically effective dose (BED) is calculated using an estimated alpha/beta ratio of 3.9 for NSCLC, we can compare this schedule to other commonly used fractionation schedules.3 The 60 Gy/30 fractions RTOG standard dose for the locally advanced NSCLC gives a BED of 90.77 Gy. The 50 Gy in 25 fractions used by the Lung Cancer Study Group randomized trial of postoperative radiation had a BED of 75.64 Gy.4 That dose was very effective in controlling microscopic or occult disease remaining after surgery in the mediastinum. Consequently, a similar BED might well be required to control similar disease within the brain. Yet the 30 Gy in 15 fractions used in RTOG 0214 has a BED of only 45.38 Gy. Therefore, it is not surprising that it was not particularly effective in achieving this objective.
Neurotoxicity
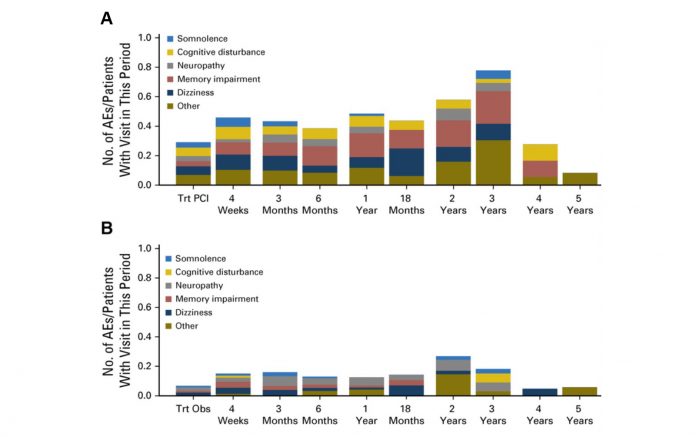
The main concern about PCI is the risk of neurotoxicity. These concerns prevented the investigators from using a higher dose. A recently published smaller randomized trial in the Netherlands presented comprehensive data regarding incidence and durability of several aspects of neurotoxicity (Fig. 1).5
Perspective on the Future of PCI
RTOG 0214 is the largest trial to address this topic. The investigators should consider amalgamating their data with data from the other trials to perform a metanalysis. At present, there is no evidence that PCI improves survival, so it cannot be recommended. Future trials testing other therapies for locally advanced NSCLC could incorporate translational analyses, which might, in theory, identify a phenotype where the risk of brain metastases is so high that PCI (or alternatively routine volumetric MRIs in follow up) might be reconsidered.6 Meanwhile, the recently reported trial of adjuvant immune therapy (PACIFIC ) suggests that immune therapy may be as effective in the brain as elsewhere in the body. In the PACIFIC trial, the incidence of brain metastases was 5.5% using consolidative durvalumab after definitive chemoradiation versus 11% without.7 ✦
About the Author: Prof. Armstrong is a professor at St. Luke’s Radiation Oncology Network, Dublin, Ireland.
References:
1. Sun A, Hu C, Gore E, et al. 10-Year Updated Analysis of NRG Oncology/RTOG 0214: A Phase III Comparison of PCI vs. Observation in Patients with LA-NSCLC. Presented at: IASLC 19th World Conference on Lung Cancer; September 23-26, 2018; Toronto, Canada.
2. Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with smallcell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476-484.
3. Santiago A, Barczyk S, Jelen U, Engenhart-Cabillic R, Wittig A. Challenges in radiobiological modeling: can we decide between LQ and LQ-L models based on reviewed clinical NSCLC treatment outcome data? Radiat Oncol. 2016;11:67
4. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. Lung Cancer Study Group. N Engl J Med. 1986 Nov 27;315(22):1377-1381
5. De Ruysscher D, Dingemans AC, Praag J, et al. Prophylactic Cranial Irradiation Versus Observation in Radically Treated Stage III Non- Small-Cell Lung Cancer: A Randomized Phase III NVALT-11/DLCRG-02 Study. J Clin Oncol. 2018;36(23):2366-2377.
6. Grinberg-Rashi H, Ofek E, Perelman M, et al. The Expression of Three Genes in Primary Non–Small Cell Lung Cancer Is Associated with Metastatic Spread to the Brain. Clin Canc Res. 2009;15(5):1755-1761.
7. Antonia S, Villegas A, Daniel D et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929