Posted: June 2017
By Cynthia L. Kryder, MS
Decades of immunology research have shown that it is possible to harness the power of the immune system to fight cancer. In particular, recent discoveries regarding the regulation of T-cell responses have contributed to the development of genomically targeted agents and immune checkpoint therapies that have improved durable responses and long-term survival in patients with lung cancer.
The ability to precisely edit genes to engineer T cells to better fight cancer is seen as a potentially revolutionary medical tool. Progress is accelerating using a gene-editing technology called CRISPRCas9.
How CRISPR Works
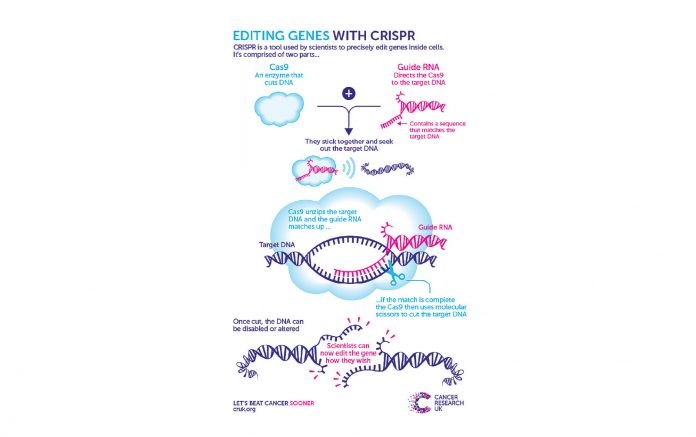
The acronym for Clustered Regularly Interspaced Short Palindromic Repeats, CRISPR, comprises two components. One is the Cas9 enzyme that acts as a molecular scissors to snip DNA. Precisely where Cas9 cuts is controlled by the second component, which is a short strand of RNA that matches up precisely with the target region of intracellular DNA. This RNA strand acts like a global positioning system to guide Cas9 to the targeted site, where it makes a cut at an exact point in the DNA sequence (Figure). Once Cas9 has cut the DNA, it is then possible to disrupt the function of a particular gene, remove it completely, introduce precise changes to the DNA sequence, or insert a completely different gene.
The power of CRISPR lies in its programmable nature. Researchers can engineer unlimited versions of the RNA strand to guide Cas9 to any gene they wish. With this specificity, scientists can target and study particular DNA sequences anywhere in the genome.
Using CRISPR to Manipulate the PD-1 Immune Checkpoint in Lung Cancer
Cell-mediated immune responses involve T cells, which become activated via 2 signals: antigen recognition and a costimulatory signal. Once activated, T cells begin to proliferate and differentiate; cytotoxic T cells eliminate cells expressing the antigen that led to their activation. Some cancers manipulate inhibitory co-signaling pathways, otherwise known as immune checkpoints, which regulate the intensity of T-cell immune responses and prevent them from attacking the wrong targets. One such checkpoint, programmed cell death 1 (PD-1), primarily works to ensure that activated T cells do not target healthy tissue near the site of an infection. The PD-1 receptor is upregulated on T cells once they become activated; its ligands, PD-L1 and PD-L2, are present in normal tissue cells. Binding of PD-1 to its ligands exerts an inhibitory effect on T cells, thereby signaling the T cell not to instigate an immune system attack.1 Thus, this checkpoint shields normal tissue from the immune attack.
Certain cancer cells protect themselves from immune attack by exploiting the PD-1 checkpoint pathway. Cancer cells have been found to express either PD-L1 or PD-L2, which binds to the PD-1 receptor on cytotoxic T cells. In the tumor microenvironment, binding of PD-1 to PD-L1 turns off the immune response necessary for tumor recognition and elimination, consequently shielding cancer cells from immune attack.1
Lu You, MD, Sichuan University, Sichuan, China, and colleagues are investigating whether it is possible to use CRISPR-edited autologous T cells to block the PD-1/PD-L1 pathway in order to improve antitumor responses in patients with metastatic non-small cell lung cancer (NSCLC).2 In this first-inhuman clinical trial, investigators will use CRISPR-Cas9 to disable the PD-1 gene on T cells. The lymphocytes will be selected and expanded ex vivo and infused back into patients. The premise is that without the PD-1 protein, the edited T cells will be able to initiate an immune attack.
Patients will be assigned to 1 of 3 treatment groups to determine the maximal tolerated dose. The primary outcome measure is the number of patients with adverse events and/or dose-limiting toxicities. Response rate, progression-free survival, and overall survival are among the secondary endpoints. Biomarkers and immunologic markers will be collected and analyzed as well.
CRISPR-edited Cells versus CAR T Cells
CRISPR-edited cells should not be confused with those that have been genetically engineered ex vivo to produce chimeric antigen receptors (CARs) on the cell surface. CARs are proteins that allow the T cells to recognize a specific antigen and to kill cancer cells that harbor the antigen on their surfaces.
CAR-modified T cells (CAR-T cells) recognize their target antigen through the scFv binding domain, resulting in T cell activation in a major histocompatibility complex-independent manner. The most widely studied application of CAR-T cell therapy targets the CD19 antigen found in B cells and has shown remarkable efficacy in B cell malignancies, particularly in anti-CD19 CAR-T cells for B cell acute lymphoblastic leukemia with up to a 90% complete remission rate.3 Similar success has not been obtained in patients with solid tumors.
Praise and Precautions
The implications of directly editing intracellular DNA to make permanent changes that affect the proteins that are produced are not fully known. Although CRISPR has been praised for its precision, DNA is complex, and it is possible for CRISPR to miss its target. In addition, CRISPR may inadvertently cut into stretches of DNA that look similar; such inaccurate editing may produce unintended results. Another concern is how modifying one gene will affect the function of other genes and molecules. Scientists will need to evaluate the effects of genetic edits in the laboratory to ensure they do not introduce genomic changes that have adverse health consequences.
Consider also that the process of extracting, genetically modifying, and multiplying cells ex vivo is a complicated and expensive process that may not be scalable. With regard to NSCLC, to justify their use, CRISPR-edited PD-1 knockout cells will need to demonstrate superior efficacy to already available anti-PD-1 antibodies.
Finally, all immunotherapies carry risk. Immune responses may be raised against normal tissues in addition to being raised against tumor cells, resulting in adverse events that may differ from adverse events commonly seen with other therapies. Adverse events reported in patients treated with immunotherapies commonly involve certain organ systems, including the skin, endocrine system, liver, gastrointestinal tract, nervous system, eyes, respiratory system, and hematopoietic cells.4
Exactly if and how the CRISPR-Cas9 gene-editing technique fits into the treatment of patients with advanced lung cancer remains to be determined.5 Although hopeful, researchers are proceeding with caution in the effort to move CRISPR-Cas9 from bench to bedside.
References
1. Zielinski C, Knapp S, Mascaux C, Hirsch F. Rationale for targeting the immune system through checkpoint molecule blockade in the treatment of non-small-lung cancer. Annals Oncol. 2013;24(5):1170-1179.
2. ClinicalTrials.gov [database]. PD-1 knockout engineered T cells for metastatic non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02793856. Accessed May 1, 2017.
3. Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53.
4. Amos SM, Duong CPM, Westwood JA, et al. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118:499-509.
5. Peel N. CRISPR gene editing: new chapter in cancer research or blot in the ethical copybook? Cancer Research UK Science Blog. Accessed February 1, 2016. http://scienceblog.cancerresearchuk.org/2016/02/01/crispr-gene-editing-new-chapter-in-cancer-research-or-blot-in-the-ethical-copybook/. Accessed May 1, 2017.