By Kara Nyberg, PhD
Posted: June 24, 2020
Genomic alterations in STK11 and KEAP1 represent two of the most common and potent contributors to a cold tumor immune microenvironment in nonsquamous NSCLC. Although prior research has shown that these mutations are tied to poor outcomes in patients treated with immune checkpoint blockade, particularly in the presence of co-occurring KRAS mutations,1 recent analysis of real-world data from the Flatiron Health Clinico-Genomic Database suggest that STK11 and KEAP1 are prognostic instead of predictive and correlate with poorer outcomes regardless of whether patients receive chemotherapy, immunotherapy, or chemoimmunotherapy.2
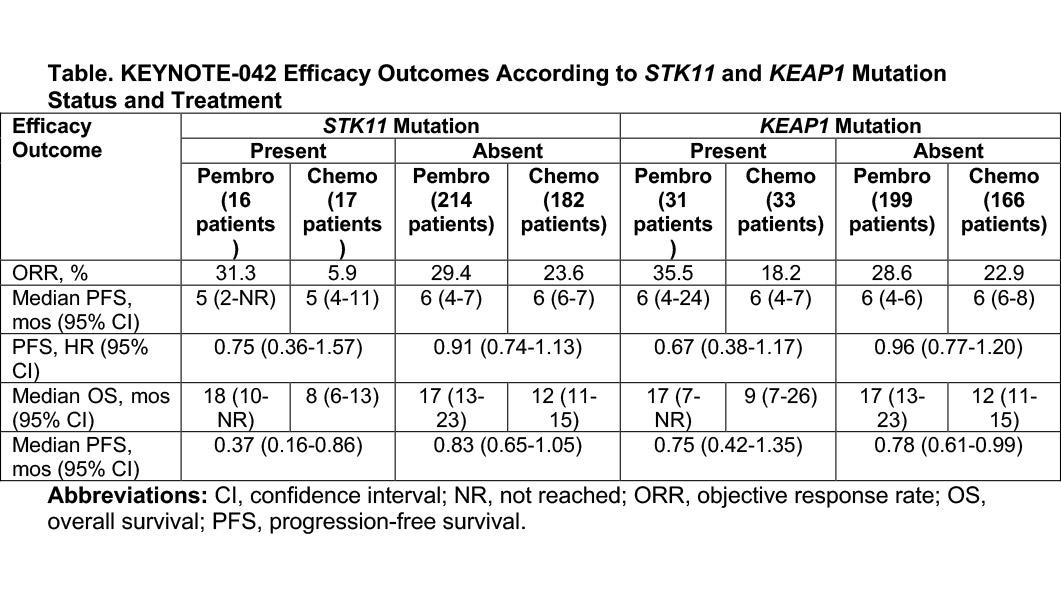
Emerging data from the KEYNOTE-042 trial, which compared pembrolizumab with platinum doublet chemotherapy in patients with PD-L1–positive NSCLC, now support this recent observation.3 Exploratory analysis of data from a subset of patients included in KEYNOTE-042 found that 23% of patients harbored STK11 and/or KEAP1 mutations. For the sake of comparison, 30% of patients in the Flatiron analysis, which included individuals with both PD-L1–positive and PD-L1–negative disease, harbored NSCLC with STK11 and/or KEAP1 mutations.
Importantly, pembrolizumab proved equally efficacious in patients with versus without either the STK11 mutation or the KEAP1 mutation based on the KEYNOTE-042 findings. In addition, pembrolizumab performed consistently better than platinum-based chemotherapy (Table). Of note, patients with STK11 mutations who received chemotherapy demonstrated particularly poor outcomes.
“Pembrolizumab monotherapy should be considered as a standard first-line treatment option for patients with locally advanced or metastatic PD-L1–positive NSCLC regardless of whether they have STK11- or KEAP1-mutant tumors,” said lead study investigator Byoung Chul Cho, MD, PhD, of the Yonsei Cancer Center, in South Korea.
Discussant Ferdinandos Skoulidis, MD, PhD, MRCP, of The University of Texas MD Anderson Cancer Center, noted that although pembrolizumab monotherapy does represent a potential treatment option for patients with STK11– or KEAP1-mutated NSCLC with 1% or greater PD-L1 expression, his preference—and likely that of others—is to use chemoimmunotherapy in such individuals. Because of this, he eagerly awaits similar subset analyses of large randomized controlled phase III trials such as KEYNOTE-189, which evaluated pembrolizumab plus chemotherapy, and IMpower150, which evaluated atezolizumab plus chemotherapy, to determine the effects of STK11 and KEAP1 alterations on clinical outcomes.
References:
1. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822‐
2.Papillon-Cavanagh S, Doshi P, Dobrin R, Szustakowski J, Walsh AM. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open. 2020;5(2):e000706.
3. Cho BC, Lopes G, Kowalski DM, et al. Relationship between STK11 and KEAP1 mutational status and efficacy in KEYNOTE-042: pembrolizumab monotherapy versus platinum-based chemotherapy as first-line therapy for PD-L1-positive advanced NSCLC. AACR Virtual Annual Meeting; April 27-28, 2020. Abstract CT084.