Posted: February 2017
By Caicun Zhou, MD
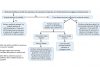
As part of an ongoing series, IASLC Lung Cancer News is exploring how drug approval processes differ country by country, and continent by continent. In China, the drug registration process includes the following steps:
• Application to the China Food and Drug Administration (CFDA) for clinical trial approval, which is similar to the Investigational New Drug Application (IND)/Clinical Trial Applications (CTA) in Western countries
• Performance of the relevant clinical trial according to the Chinese Good Clinical Practice (GCP) and Regulations so as to secure Chinese patients’ data and clinical study report
• Application to the CFDA for marketing authorization, e.g., Imported Drug License (IDL) or Local Production Permit, a process similar to the New Drug Authorization (NDA) or Biological License Authorization (BLA)/Marketing Authorization Applications (MAA) used in Western countries
The Center for Drug Evaluation (CDE) under the CFDA is responsible for technical review, and the CFDA takes charge of administrative review and final approval.
The review and approval timeline defined in the current regulations is theoretical. …. actual review and approval timelines are longer and vary from product to product.
During IND/NDA review, CFDA is empowered by regulations to perform onsite (research site, clinical site/GCP and manufacturing site/GMP) inspection. The on-site inspections are mandatory for local manufacturers, but for imported drugs applications inspections occur only as needed.
The review and approval timeline defined in the current regulations is theoretical. If there are no formal queries during the process, the timeline is usually about 125 days. However, from a practical point of view, because of the heavy workload in CDE due to a shortage of review resources compared with a large volume of applications, the actual review and approval timelines are longer and vary from product to product.
Ultimately, the CFDA is responsible for protecting the public health by ensuring the safety and efficacy of medical products and medical devices.
Recent Changes and Future Trends
In Aug 2015, The State Council, the Chief administrative authority of the People’s Republic of China, issued directives to reform the review and approval system, including accelerating the review and approval process for development and marketing authorization of new drugs with clinical value and generic drugs with clinical value and urgent need.
Accordingly, the CFDA took major measures on the review and approval process and system reform as follows:
• Priority Review and Approval: this applies to applications with obvious clinical value, for example, the application for drugs preventing or treating AIDS, TB, viral hepatitis, orphan diseases, malignant tumors, pediatric disease, and other serious diseases
• Communications on drug R&D and scientific review with CDE: the CFDA has published the relevant guideline to define the formal meeting type, the process, requirement, and timeline for consulting and preparation, which will grant more communication opportunities to industry
• Improvement on clinical data quality: the CFDA has requested preapproval GCP-inspection for all NDAs (both for imported drugs and local production) from the end of 2015 With these activities and reform measures, the CFDA hopes to encourage drug innovation, standardize evaluation and drug approval, and improve drug quality.