By Prof. Corinne Faivre-Finn
The optimal timing and schedule of thoracic radiation in the management of limited-stage small cell lung cancer (LS-SCLC) continues to provoke debate. Since the publication of Intergroup 0096 in 1999, there had been controversy about the standard chemo-radiotherapy (cCTRT) regimen in LS-SCLC.1 Although twice-daily (BD) radiotherapy (RT) was associated with a higher survival compared to once-daily (OD) RT, concerns regarding toxicity (i.e., a third of the patients developing grade 3 or more esophagitis), together with logistical issues in the delivery of BD RT and criticism about the low dose of RT used in the control arm of Intergroup 0096, led to the limited adoption of this regimen in routine practice.2
The CONVERT trial is the first multicenter, international, randomized phase III trial aiming to establish a standard chemo-radiotherapy regimen in LS-SCLC. It is the largest-ever trial completed in this group of patients. We reported the trial results at the 2016 Annual Meeting of the American Society of Clinical Oncology.3
In CONVERT, patients with LS-SCLC were randomized 1:1 to receive either 45 Gy in 30-BD fractions over 3 weeks or 66 Gy in 33-OD fractions over 6.5 weeks, starting on day 22 of cycle 1 chemotherapy (four to six cycles of cisplatin and etoposide, according to investigator’s prespecified choice), followed by prophylactic cranial irradiation, if indicated. RT was planned using either 3-D conformal or intensity-modulated radiation therapy (IMRT). The primary endpoint of the study was 2-year survival and all analyses were by intention to treat. The study enrolled 547 patients recruited from 73 centers in seven European countries and Canada between 2008 and 2013.
Patient characteristics were well balanced in both arms of the study. Median age was 63 years (15% were older than 70 years), almost 50% were female, and the majority of patients had a performance status of 0 or 1 and were ex-smokers or current smokers.
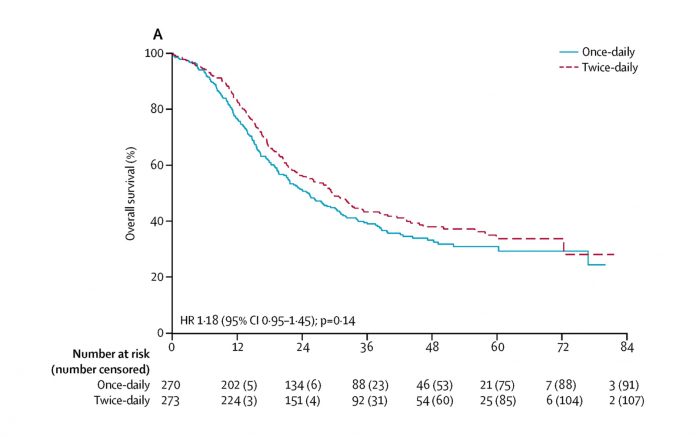
At a median follow-up of 45 months, 2-year survival was 56% compared to 51%, and median overall survival was 30 months compared with 25 months in the BD RT and the OD RT arms, respectively, a difference that did not prove to be statistically significant. Furthermore, no statistically significant differences between the two groups were reported in terms of local or metastatic disease progression.
Acute toxicity rates were not significantly different between the two groups, with the exception of more neutropenia in patients treated with BD RT. Nor was there any difference in terms of acute esophagitis or pneumonitis. There was one death in the BD group and two in the OD group due to radiation pneumonitis. Few patients developed severe late toxicity.
In conclusion, OD RT did not result in a superior survival or worse toxicity than BD RT. The survival for both regimens was higher than previously reported, possibly due to more frequent PET/CT staging, and radiation toxicities were lower than expected, likely due to using modern RT techniques. The implications of CONVERT are important. The OD arm did not show superior survival as originally postulated. Because CONVERT was not an equivalency trial, the only study to date that has shown superiority for one RT regimen over another in LS-SCLC was the Intergroup 0096 trial; because there were no major differences in toxicity, 45 Gy in 30-BD fractions should continue to be regarded as standard of care. However, OD RT at a dose of 66 Gy in 33 fractions can certainly be considered an alternative regimen if 45 Gy in 30-BD fractions cannot be delivered due to patients’ choice, departmental logistics, or other factors.
References
1. Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999; 340:265–271.
2. Komaki R, Khalid N, Langer CJ, et al. Penetration of recommended procedures for lung cancer staging and management in the United States over 10 years: a quality research in radiation oncology survey. Int J Radiat Oncol Biol Phys. 2013; 85:1082-1089.
3. Faivre-Finn C, Snee M, Ashcroft A, et al. CONVERT: An international randomised trial of concurrent chemo-radiotherapy (cCTRT) comparing twice-daily (BD) and once-daily (OD) radiotherapy schedules in patients with limited stage small cell lung cancer (LS-SCLC) and good performance status (PS). J Clin Oncol. 2016; 34 (15) suppl: 8504.