By Vassiliki A. Papadimitrakopoulou, MD
Lung-MAP (S1400), is a unique master multi-study protocol that incorporates genomic testing of tumors through a next-generation sequencing (NGS) platform (Foundation Medicine) and biomarker-driven therapies for patients with squamous cell lung cancer (SCC) after progression on first-line therapy. This effort represents a unique private-public partnership supported by the National Cancer Institute (NCI) and the National Clinical Trials Network (NCTN), the Foundation for the National Institutes of Health (FNIH), and Friends of Cancer Research (FOCR). The overall aim of the Lung Master Protocol is to find new agents and companion diagnostics for precisely defined molecular subsets of patients with advanced squamous cell cancer, for whom there are few effective therapeutic options and a major need for new targeted drugs. With the exception of the newly approved nivolumab and pembrolizumab, in the second-line setting, which occurred after the launch of Lung-MAP in June of 2014, and necitumumab in the first-line setting, there have been few other FDA approvals specifically for squamous lung cancer.
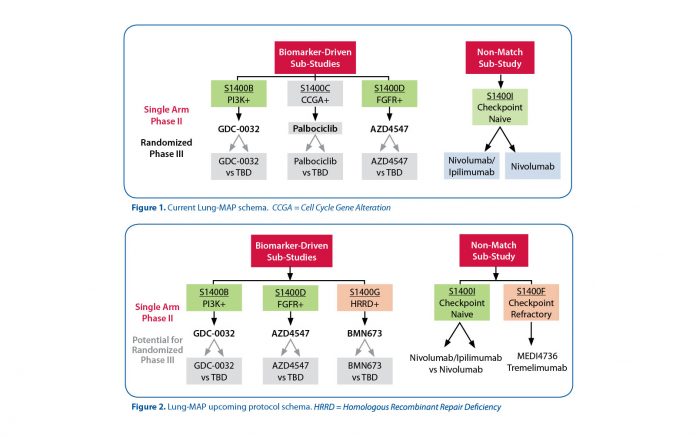
Research studies like The Cancer Genome Atlas (TCGA) have detected a large number of somatic gene mutations/amplifications in patients with this disease. Many of these are targetable by investigational agents (examples: PIK3CA, BRAF, FGFR2 are mutated while EGRF and MET are amplified). However, the frequency of these changes in these patients is low (5%–20%), making recruitment and treatment very challenging in the traditional single-agent trial setting. Hence, the Lung- MAP strategy is to use a common platform (Next Generation DNA Sequencing) to enable a single “umbrella screening protocol” to efficiently find patients with diverse uncommon molecular changes. According to their profile, these patients are assigned to a “matched” trial with an investigational drug or combination of drugs for the target. Thus, the Master Protocol has a single entry point under its “umbrella” but then allows for multiple simultaneous studies with a single Institutional Review Board (IRB) approval. Based on the need for study assignment of patients whose tumor does not harbor targetable alterations and the rapid therapeutic establishment of the efficacy of immunotherapy, a non-match trial that tests nivolumab plus ipilimumab vs nivolumab alone has been included in the umbrella protocol. Current biomarker-driven studies include: S1400B evaluating taselisib, a PI3K inhibitor, and S1400D evaluating AZD4547, an FGFR inhibitor, while S1400A evaluating the activity of durvalumab, an anti-PD-L1 antibody, was closed in December 2015 and S1400C evaluating palbociclib, a CDK 4/6 inhibitor, was closed in September of 2016 (Figure 1).
As of September 2016, 1,003 patients were registered to S1400, with accruals from ALIIANCE, ECOG-ACRIN, NRG, and the leading group SWOG, while 723 patients were notified of their sub-study assignment and 364 patients were registered to a sub-study. Principal investigators from all cooperative groups are leading the individual studies (see Acknowledgement). Patients can enter the trial either at progression on prior therapy or can be prescreened while they are receiving front-line therapy and prior to progression with the option to request study assignment at progression.
Several sub-studies are currently under development, including a study exploring the activity of talazoparib, a PARP inhibitor for patients with homologous recombination deficient tumors, and a study exploring the activity of the combination of durvalumab and tremelimumab for patients with immunotherapy relapsed or refractory tumors as shown in the schema (Figure 2). Genomic screening is feasible in the majority of patients, and findings so far are confirming the anticipated prevalence of targeted alterations. ✦
Acknowledgment. The leadership team for this study includes: Roy Herbst, MD, PhD; Mary W. Redman, PhD; David R. Gandara, MD; Fred R. Hirsch, MD, PhD; Philip C. Mack, PhD; Hossein Borghaei, DO; Corey J. Langer, MD; James L. Wade, MD; Martin Edelman, MD; Kathy Albain, MD; Primo Lara Jr, MD; Charu Aggarwal , MD; Mark A. Socinski, MD; Scott N. Gettinger, MD; Lyudmila A. Bazhenova, MD; Shakun Malik, MD; Vince Miller, MD; Ellen V. Sigal; and Karen Kelly, MD.