By Anjali Saqi, MD, MBA; Deepali Jain, MD, FIAC; Lukas Bubendorf, MD; Keith Kerr, MB, ChB, MRCPath, FRCPath, FRCP(Ed); and Andre Moreira, MD, PhD
Posted: December 11, 2019
Lung cancer is a leading cause of cancer-related deaths worldwide. In the 21st century, there have been two significant developments in the systemic management of lung cancers. The first was the introduction of tyrosine kinase inhibitors (TKIs) reserved for patients with confirmed driver mutations. The second, as well as the most recent, is the incorporation of immune checkpoint inhibitors as a standard of care in the armamentarium. There are multiple immune checkpoint inhibitors, and each has its respective predictive PD-L1 immunohistochemical (IHC) biomarker test—companion or complementary— that interrogates patient eligibility.
Although promising data have continued to expand indications for immune checkpoint inhibitors, enrollment into clinical trials is frequently restricted to those with biopsy/histology specimens. This criterion affects clinical adoption and may cause question over the validity of testing on cytology samples, which comprise a significant proportion and are often the only available specimens upon which lung cancer diagnoses are rendered. We believe that cytology-type samples, when prepared appropriately, are valid material for clinical PD-L1 testing.
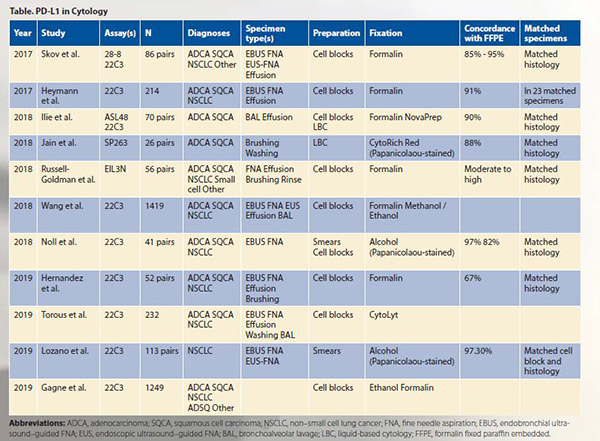
In an effort to address the shortcomings and lack of data, several studies across different laboratories and countries have examined PD-L1 testing on cytology specimens, including as part of the Blueprint Phase 2 Project that compared staining of five PD-L1 IHC assays on clinical samples.1-12 These studies have addressed several questions, which are summarized here.
1. Can cytology specimens be used for PD-L1 testing?
The overall consensus is that cytology specimens are suitable for PD-L1 testing.
2. Are results of PD-L1 cytology specimens equivalent to those of surgical biopsies and resections?
Results of PD-L1 testing on cytology specimens are highly concordant with those of histology specimens, including among squamous cell carcinomas, adenocarcinomas, and NSCLCs.
3. Which PD-L1 assays can be used on cytology preparations?
All assays have been tested on cytology specimens. Akin to testing on their histology counterparts, cytology specimens with assays 22C3, 28-8, and SP263 are similar, whereas SP142 and 73-10 demonstrate relatively lower and higher sensitivities, respectively, for tumor cell staining.
4. What types of cytology specimens can be stained with PD-L1?
PD-L1 testing is feasible on all cytology preparations, including fine needle aspirations (FNAs) and exfoliative specimens (i.e., effusions, bronchoalveolar lavages [BAL], brushings) used in the diagnosis and staging of lung cancer.
5. Which cytology preparations have been evaluated for PD-L1?
Most published PD-L1 studies on cytology specimens, including as part of the Blueprint Phase 2, have been performed on cell blocks. Although concordant with matched histology specimens and promising, there are limited data on the use of Papanicolaou-stained slides (i.e., smears and liquid-based cytology [LBC]) relative to cell blocks.
6. What are the advantages of cytology specimens?
First and foremost, cytology specimens represent a significant proportion of lung cancer specimens and, therefore, provide greater access to patients for potential systemic therapy options. Second, the back-and-forth or fanning tissue-disruptive motion of FNA acquisition is advantageous for sampling a broader area than is feasible with a relatively localized sampling with a biopsy. Similarly, effusions and BALs can detect cells from different areas. These sampling modalities may capture tumor heterogeneity. An advantage specific to cell blocks is preservation of some degree of architecture. This is particularly helpful for matching and localizing cells of interest on serial slides, identification of cell membrane staining, as well as providing histological cues for those without formal training or limited exposure to interpreting cytology.
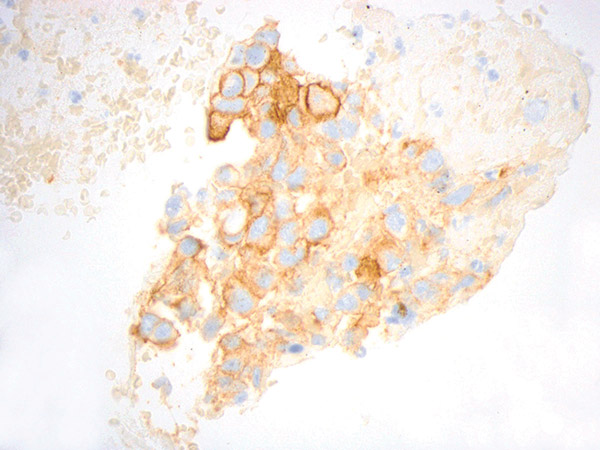
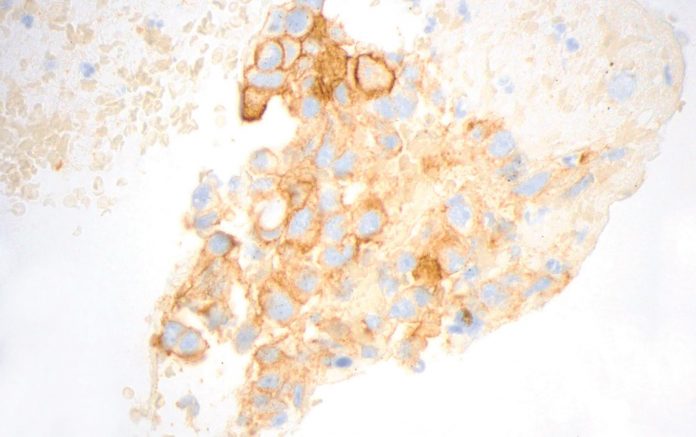
Figure courtesy of Dr. John Crapanzano
7. What are potential drawbacks of cytology specimens?
Several limitations apply to all small specimens (small biopsy and cytology) rather than specifically to cytology. Most predictive assays frequently have minimum tumor cell/tissue requirements. Small specimens may suffer from low yield and insufficient cellularity following tissue allocation for various tests set forth by guidelines for advanced-stage lung adenocarcinomas. When interpreting PD-L1 results, differentiating between relatively small tumor cells without significant nuclear pleomorphism and macrophages, especially when both are similar in size and singly dispersed, can be challenging. Some PD-L1 assays require tabulation of immune cells; there is poor reliability on histology specimens. Moreover, in a limited sample, assessing whether immune cells are associated with the tumor or not may not be feasible and should be evaluated only in the context of sufficient and intact tissue fragments.
Figure courtesy of Dr. John Crapanzano
There are drawbacks unique to cytology specimen subtypes other than formalin-fixed paraffin embedded (FFPE) cell blocks, including cell blocks with ethanol pre-fixation, smears, and LBC. First, on smears and LBC, membranous staining may be less pronounced, and the distinction between membranous and cytoplasmic staining could be blurred and compromised by overlapping cells. Also, non-FFPE preparations require additional rigorous validation and possible modifications of protocols and workflows, which may result in suboptimal adoption by laboratories.
8. Which type of cytology preparation should be used for PD-L1 staining?
FFPE cell blocks are currently the recommended cytology preparations for PD-L1 testing based on the greatest available data, including part of the Blueprint Phase 2 comparability study. Moreover, use of a standardized method that closely parallels histology provides initiative to integrate cytology into clinical trials, perform interlaboratory comparisons and outcomes analyses, digitally scan/evaluate slides without a z-axis, and incorporate other efforts frequently restricted to histology specimens.
9. What are possible future directions?
Dual/multiplex staining that highlights and differentiates the tumor cells and macrophages can potentially aid in a more accurate assessment. The role of digital analysis and automated scoring requires further exploration. Most importantly, incorporation of FFPE cell blocks into clinical trials is essential. ✦
About the Authors: Dr. Saqi is a professor in the Department of Pathology and Cell Biology at Columbia University Medical Center. Dr. Jain is an additional professor in the Department of Pathology at All India Institute of Medical Sciences, New Delhi, India. Prof. Bubendorf is a professor of Pathology at University Hospital Basel, Switzerland. Prof. Kerr is a consultant pathologist in the Department of Pathology, Aberdeen University Medical School and Aberdeen Royal Infirmary, UK. Prof. Moreira is a professor of Pathology at New York University Langone Medical Center, where he also serves as director of Surgical Pathology and CardioThoracic Pathology service and director of the Center of Biorepository Research and Development.
References:
1. Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol. 2017;125(12):896-907.
2. Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol. 2017;25(7):453-459.
3. Ilie M, Ngo-Mai M, Long-Mira E, et al. Using 22C3 Anti-PD-L1 Antibody Concentrate on Biopsy and Cytology Samples from Non-small Cell Lung Cancer Patients. J Vis Exp. 2018(139).
4. Jain D, Sukumar S, Mohan A, Iyer VK. Programmed death-ligand 1 immunoexpression in matched biopsy and liquid-based cytology samples of advanced stage non-small cell lung carcinomas. Cytopathology. 2018;29(6):550-557.
5. Noll B, Wang WL, Gong Y, et al. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol. 2018;126(5):342-352.
6. Russell-Goldman E, Kravets S, Dahlberg SE, Sholl LM, Vivero M. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathol. 2018;126(4):253-263.
7. Torous VF, Rangachari D, Gallant BP, Shea M, Costa DB, VanderLaan PA. PD-L1 testing using the clone 22C3 pharmDx kit for selection of patients with non-small cell lung cancer to receive immune checkpoint inhibitor therapy: are cytology cell blocks a viable option? J Am Soc Cytopathol. 2018;7(3):133-141.
8. Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol. 2018;13(9):1302-1311.
9. Wang H, Agulnik J, Kasymjanova G, et al. Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol. 2018;29(6):1417-1422.
10. Gagne A, Wang E, Bastien N, et al. Impact of Specimen Characteristics on PD-L1 Testing in Non-Small Cell Lung Cancer: Validation of the IASLC PD-L1 Testing Recommendations. J Thorac Oncol. 2019. S1556-0864(19)33186-33187.
11. Hernandez A, Brandler TC, Zhou F, Moreira AL, Schatz-Siemers N, Simsir A. Assessment of Programmed Death-Ligand 1 (PD-L1) Immunohistochemical Expression on Cytology Specimens in Non-Small Cell Lung Carcinoma. Am J Clin Pathol. 2019;151(4):403-415.
12. Lozano MD, Abengozar-Muela M, Echeveste JI, et al. Programmed death-ligand 1 expression on direct Pap-stained cytology smears from nonsmall cell lung cancer: Comparison with cell blocks and surgical resection specimens. Cancer Cytopathol. 2019;127(7):470-480.