By Mari Mino-Kenudson, MD, and Sylvie Lantuejoul, MD, PhD
Posted: November 12, 2019
Determination of PD-L1 expression by immunohistochemistry (IHC) has been widely evaluated in clinical trials as a predictive biomarker for patients with advanced NSCLC and is routinely performed to determine eligibility for pembrolizumab therapy, either as monotherapy or in combination with chemotherapy or, in Europe, for durvalumab therapy after chemoradiation for patients with unresectable stage III NSCLC. Four PD-L1 commercial assays validated in clinical trials have been approved by the U.S. Food and Drug Administration or the European Medicines Agency or are used as in vitro diagnostic tests in multiple countries; costs have limited their use, however, leading to the use of laboratory-developed tests (LDTs). Overall, PD-L1 testing is not uniformly implemented across different geographic regions and across different laboratories. Although PD-L1 expression determined by IHC is not a perfect biomarker, implementation of uniform standards to improve its predictive performance is warranted.
With this background, the Immune Biomarker Working Group of the IASLC Pathology Committee conducted an international online survey between February 1 and May 31, 2019 on PD-L1 IHC testing for patients with NSCLC. The goals of the survey were to assess the prevalence of and process for PD-L1 testing and to identify issues to improve the practice globally. The survey included more than 20 questions on pre-analytical, analytical, and post-analytical aspects of PD-L1 IHC testing, including the availability/type of PD-L1 IHC assay(s), participation in a quality-assurance (QA) program, and completion of training.
Illuminating Global Inconsistencies
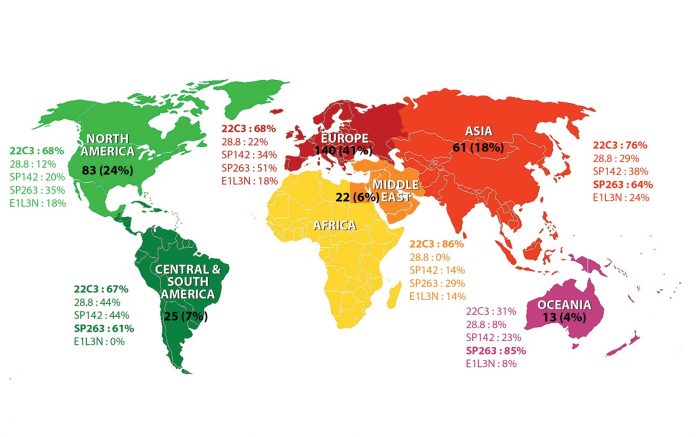
A total of 344 pathologists from 310 institutions in 64 countries participated in the survey: 140 (41%) from Europe, 83 (24%) from North America, 61 (18%) from Asia, 25 (7%) from the Central/South America, 22 (6%) from the Middle East/Africa, and 13 (4%) from Oceania (Figure). Of these, 32% primarily practice thoracic pathology, 30% practice both thoracic pathology and cytology, 6% practice primarily cytology, and 29% practice general pathology (3% defined their primary practice as “other”). Of note, a small fraction of participants (2.9% from nine countries) do not perform PD-L1 IHC, and another 9.9% send out samples to other laboratories—in particular, 25% of respondents from North America and 15% from Central/South America outsource their samples. Regarding the specimen type, although cytology specimens have not been validated in trials as samples for PD-L1 testing and the PD-L1 IHC scores on cytology samples are particularly subject to interobserver variability,1 cell blocks and cytology smears are used by 72% and 10% of all participants, respectively, along with biopsies and surgical resections (94% and 89%, respectively). Among PD-L1 antibody clones, 22C3 is most frequently used of 69% of all respondents with the clinical-trial validated, commercial assay in 60% of the laboratories conducting 22C3 PD-L1 IHC. The SP263 assay was used by 51% of respondents; 28.8 and SP142 assays are used by only 21% and 31% of respondents, respectively (Figure). The numbers appear to reflect the regulatory approval status of PD-1 and PD-L1 agents for various indications and the subsequent requirement of use of a PD-L1 IHC assay for each indication. In Central/South America, Europe, Asia, and Oceania, the majority of laboratories run SP263 IHC (61%- 85%) commonly with the commercial assay, likely reflecting the fact that > 70% of the laboratories are equipped with Ventana automation in those regions. Conversely, the SP263 clone is used only in about one-third of the laboratories in North America and in the Middle East/Africa.
The vast majority of laboratories have external QA measures in place, but 18% report a lack of QA. However, only 63% of respondent laboratories participate in a formal QA program; this is a more frequent practice in Europe (72%) and Oceania (77%). PD-L1 testing guidelines are applied in the vast majority of laboratories (96%), but national or local guidelines are used only by 62%—mainly in North America (73%), Europe (68%), Asia (61%), and Oceania (54%). Conversely, the IASLC Atlas of PD-L1 Testing in Lung Cancer is the only “guideline” referenced in 76% (Central/ South America) and 55% (the Middle East/Africa) of laboratories. It has been reported that interobserver variability may be higher than interassay viability.2 Thus, training for scoring of PD-L1 IHC appears to be very important to improve interobserver concordance. Although 84% of all respondents have undergone some training, the rate is lower in the North America (69%), Central/South America (64%), and in the Middle East/Africa (67%).
The median turn-around time (TAT) for results is 1-2 days in North America, Europe, and Oceania, 2-3 days in Asia and Central/South America, and 3-4 days in the Middle East/Africa. TAT is the shortest in Europe. In North America, laboratories that outsource PD-L1 testing report longer TAT.
Although the majority of respondents noted that they use a standardized report with no significant difference between regions, 14% of laboratories report the results of PD-L1 IHC with a free text.
There is heterogeneity in PD-L1 testing practice across regions, as well as across individual laboratories. The regional differences appear significant in PD-L1 testing status, PD-L1 antibody clones/assays used, training, the availability of local or national guidelines, and TAT. In addition, a significant minority of respondents reported a lack of QA, in particular, formal QA, formal training, and/or a standardized reporting system. New actions encouraging formal QA participation, application of standardized reporting, and implementation of regional training (particularly on cytologic samples) are expected, and harmonization of LDTs must be achieved at a global level. The IASLC Pathology Committee members encourage that laboratories offering PD-L1 testing to participate in formal QA, apply a standardized reporting format, and attend regional training. ✦
About the Authors: Dr. Mino-Kenudson is director of the Pulmonary Pathology Service, Department of Pathology at Massachusetts General Hospital and professor of pathology at Harvard Medical School. Dr Lantuejoul is consultant in the Department of Biopathology at the Centre Léon Bérard, Lyon and professor of biopathology at the Grenoble Alpes University, France.
References:
1. Kerr KM, Tsao MS, Yatabe Y, et al. Phase 2B of Blueprint PD-L1 immunohistochemistry assay compatibility study. WCLC 2018 abstract OA03.03.
2. Brunnström H, Johansson A, Westbom-Fremer S, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod Pathol. 2017;30:1411-1421. Acknowledgement: The authors thank Dr. Nolwenn Le Stang from the Biopathology Department of Centre Léon Bérard, Lyon France for her statistical assistance and contribution of the figure.
Acknowledgement: The authors thank Dr. Nolwenn Le Stang from the Biopathology Department of Centre Léon Bérard, Lyon France for her statistical assistance and contribution of the figure.