By Kenneth Michael Cummings, PhD, MPH
The US FDA recently finalized a new rule (http://www.fda.gov/TobaccoProducts/ Labeling/RulesRegulationsGuidance/ ucm388395.htm) that will extend its regulatory authority to all tobacco products, including small and large cigars, hookah tobacco (also called waterpipe tobacco), pipe tobacco, nicotine gels, dissolvable tobacco products, and electronic cigarettes (e-cigarettes).
What will the new rule do?
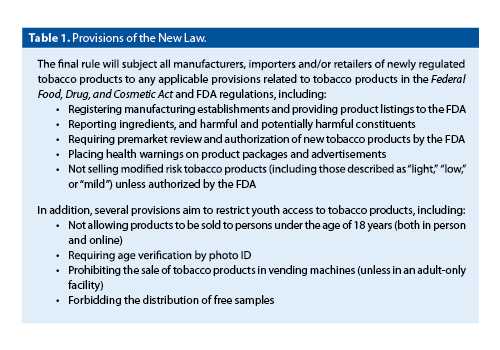
This new rule builds on groundwork, established in June 2009, giving the FDA authority to regulate cigarettes, cigarette tobacco, roll-your-own tobacco (RYO), and smokeless tobacco products, after Congress passed and the President signed the Family Smoking Prevention and Tobacco Control Act. This Act gave the agency authority to regulate the manufacturing, distribution, and marketing of tobacco products. The new regulations extend FDA authority to a wider range of tobacco products. Table 1 provides a quick summary of what the new tobacco rule will do.
Why did the FDA take this action?
Before the FDA finalized a rule deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, tobacco products such as small and large cigars, pipe tobacco, hookah tobacco, nicotine gels, dissolvable tobacco products, and e-cigarettes could be sold without any review of their ingredients, how they were made, or their potential dangers. In addition, there was no federal law stopping retailers from selling e-cigarettes, hookahs, or cigars to youth under the age of 18. This new rule will allow the FDA to better fulfill its mandate to improve public health and to protect future generations from the risks of tobacco use. The tobacco product review process allows the FDA to evaluate important factors such as ingredients, product design, and health risks, as well as the appeal of products to youth and non-users.
What’s the timeline?
Scheduled to take effect August 8, 2016, the recent rule “deems” all products meeting the statutory definition of tobacco product, including components or parts, to be subject to the FDA’s tobacco product authorities. The new deeming rule requires manufacturers of newly regulated tobacco products that were not on the market as of February 15, 2007, to show that products meet the applicable public health standard set by the law. After the new deeming rule takes effect on August 8, the FDA expects that manufacturers of these newly deemed products will continue selling their products for up to 2 years while they submit product applications.
The FDA anticipates it will take them about 1 year to review the product applications before issuing a marketing authorization, where appropriate. Of course, the timeline could change, as legislation has already been filed that may exclude some products (premium cigars) and change the effective date for defining a new tobacco product from February 15, 2007 to August 8, 2016. In addition, lawsuits have already been filed against the FDA challenging various provisions of the new deeming rule, which could further delay FDA actions.
What’s the bottom line?
Public health groups have been united in their view that the FDA should have the authority to regulate all tobacco products, not just cigarettes, RYO, and smokeless tobacco, as has been the case since the 2009 Family Smoking Prevention and Tobacco Control Act. That said, there remains a vigorous debate within the public health community as to how to implement the new tobacco rule. While there has been a decline in cigarette use by teens over the past decade, the increasing use of small cigars, hookahs, and e-cigarettes by teenagers has caused some public health groups to urge the FDA to aggressively restrict the marketing of these products, especially the use of flavoring agents that may make these products appealing to youth. Other public health groups have argued that the FDA ought to apply a light touch when it comes to regulating noncombustible tobacco products such as dissolvable tobacco products and e-cigarettes, since these products could conceivably serve as safer substitutes for combustible tobacco products, especially cigarettes. By treating all tobacco products the same from a regulatory prospective, some have worried that this would stifle development of safer products, unfairly favoring well-resourced tobacco manufacturers in meeting the FDAs regulatory hurdles, and potentially misleading consumers into believing that all tobacco products are equally risky, which is not the case.
In the end, it is good that FDA has been given the authority to regulate all tobacco products. However, what this will ultimately mean in terms of public health benefit will depend in large measure by how the FDA choses to implement this authority. Will the FDA recognize the continuum of risk that obviously exists between different classes of tobacco products and tailor its regulations accordingly, or will it instead apply its regulatory authority in a way that assumes all tobacco products are the same? Only time will tell.
Kenneth Michael Cummings, PhD, MPH, is co-leader of the Tobacco Research Program at the Hollings Cancer Center, Medical University of South Carolina, US.











