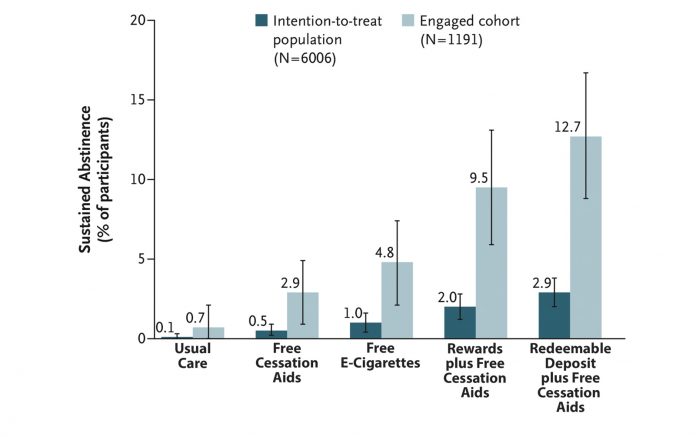
Reproduced with permission from Halpern SD et al. N Engl J Med. 2018;278:2302-2310.
By Raymond Niaura, PhD
Posted: April 1, 2019
IN REFERENCE TO: Halpern SD, French B, Small DS, et al.
Randomized trial of four financial incentive programs for smoking cessation. N Engl J
Med. 2015;372:2108-2117.

As has been true for decades, smoking remains the leading cause of preventable morbidity and mortality in the United States.1 Smoking prevalence continues to decline, but approximately 14% of adults still smoke cigarettes regularly (34.3 million).2 First-line, U.S. Food and Drug Administration–approved smoking cessation treatments (e.g., varenicline, bupropion, and nicotine replacement therapy [NRT]) are effective for approximately 20% of smokers after 1 year,3 but they remain underutilized.4 Workplace interventions can reach large numbers of smokers, and financial incentives to quit smoking, delivered via workplace smoking-cessation programs, have shown some promise.5,6 For example, financial incentive programs (up to $800), contingent on biochemically verified quitting, resulted in superior sustained 6-month quit rates (9.4% to 16.0%) compared with usual care (6.0%).6 These studies, however, report results only for those motivated smokers who volunteered and engaged in the programs, and they did not test the potential combined efficacy of financial incentives and other approaches (e.g., cessation aids such as NRT).
Gathering Data
The most recent study by Halpern and colleagues tested the efficacy of separate and combined treatments including financial incentives and the offer of free e-cigarettes or NRT patches, gum, and lozenges.7 The results present a mixed picture. Only 19.8% of smokers informed about the study (1,191 of 6,006) engaged in the trial, logging in at least once onto the trial website (another 125 opted out before random selection). The intent-to-treat analyses showed that sustained smoking abstinence at 6 months ranged between 0.1% for usual care and 2.9% for smokers who participated in the redeemable deposit incentive program along with access to free cessation aids (Fig.). Although smokers in the financial incentive group were statistically significantly more likely to achieve abstinence, overall low quit rates call into question the practical significance of these findings. The pattern of findings was similar, but quit rates overall were higher when data only from smokers who engaged in treatment were analyzed.
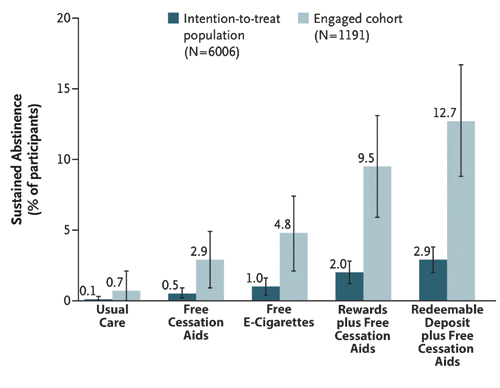
Therefore, it seems fair to conclude that financial incentives added to free cessation aids (mostly NRT) can augment quit rates compared to free cessation aids alone. Left unanswered, however, is whether incentives can also boost quit rates when combined with free e-cigarettes. Smokers offered free e-cigarettes are more likely to quit compared to those offered NRT, although not significantly so. More important than the treatment effects, perhaps, are the overall low rates of treatment engagement (19.8%) and low rates of incentive treatment acceptance, defined as agreeing to the incentive contract (51.2%). Smokers, however, were more likely to accept the external monetary reward-based incentive programs (90.0%) than the monetary self-deposit based reward programs (13.7%). More research is required to determine why smoking cessation treatments, even those that are free, remain overwhelmingly underutilized when offered in workplace settings. ✦
About the Author: Dr. Niaura is a professor of Social and Behavioral Sciences at New York University.
References:
1. Ma J, Siegel RL, Jacobs EJ, Jemal A. Smoking-attributable Mortality by State in 2014, U.S. Am J Prev Med. 2018;54(5):661-670.
2. Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225-1232.
3. Rosen LJ, Galili T, Kott J, Goodman M, Freedman LS. Diminishing benefit of smoking cessation medications during the first year: a meta-analysis of randomized controlled trials. Addiction. 2018;113:805-816.
4. Rodu B, Plurphanswat N. Quit Methods Used by American Smokers, 2013-2014. Int J Environ Res Public Health. 2017;14(11).
5. Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360:699-709.
6. Halpern SD, French B, Small DS, et al. Randomized trial of four financial incentive programs for smoking cessation. N Engl J Med. 2015;372:2108-2117.
7. Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A Pragmatic Trial of E-Cigarettes, Incentives, and Drugs for Smoking Cessation. N Engl J Med. 2018;378(24):2302-2310.










