By Erik J. MacLaren, PhD
The growing number of checkpoint inhibitors currently being developed, without question, has ushered in a new era in clinical oncology, particularly in the treatment of thoracic malignancies.These agents block various components of the immune checkpoint pathway, such as programmed cell death-1 (PD-1) receptor, which is expressed on the surface of immune cells, and its ligand PD-L1, which is frequently expressed on tumor cells and allows them to evade immune surveillance. By blocking checkpoint signaling, these drugs unleash the power of the patient’s own immune system against malignancies. Drugs targeting PD-1 have moved into the clinic in recent years for use in patients with melanoma, and in
2015, the FDA approved the first two immunotherapeutic agents for use in lung cancer.
The PD-1 inhibitor nivolumab (Opdivo) received approval in March 2015 for use in patients with advanced squamous non-small cell lung cancer (NSCLC) whose disease has progressed on platinum-based chemotherapy. In October 2015, approval was expanded
to advanced non-squamous NSCLC as well. That month, the FDA approved another PD-1 antibody for the treatment of NSCLC, pembrolizumab (Keytruda).
The success of these anti-PD-1 drugs in NSCLC has also
led to a flurry of interest in other checkpoint inhibitors.
Nivolumab was also approved last year by the European Medicines Agency for second-line treatment of squamous NSCLC.
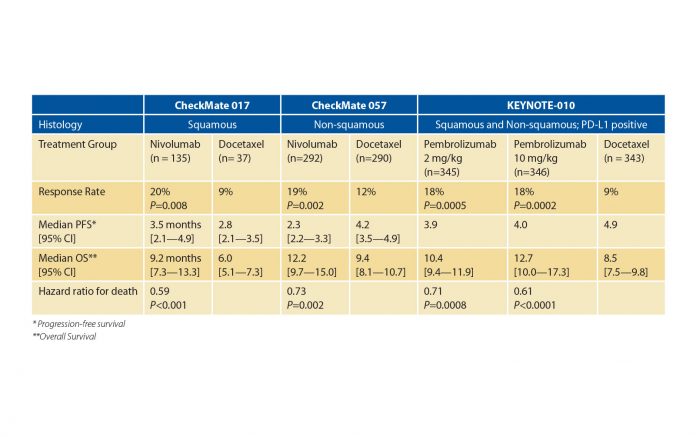
Results from two phase 3 trials, CheckMate 0171 and CheckMate 057,2 demonstrated that second-line nivolumab prolonged overall survival (OS) compared to docetaxel in an unselected population of patients with squamous and non-squamous NSCLC, respectively; and the phase 3 KEYSTONE-0103 trial showed that pembrolizumab increased OS compared to docetaxel in patients with PD-L1 positive NSCLC (Table 1). Importantly, not only did these studies establish that nivolumab and pembrolizumab prolonged patient survival, but they were also associated with far fewer adverse events and improved quality of life compared to docetaxel.
These positive results in both histologically determined, otherwise-unselected populations have raised questions about the predictive power of PD-L1 as a biomarker. Data from the nivolumab trials showed that PD-L1 expression levels were positively correlated with treatment benefits in non-squamous NSCLC with respect to response rates and OS, but not in squamous NSCLC. Consequently, work continues to better define the optimal role for PD-L1 expression in patient selection.
The success of anti-PD-1 drugs in NSCLC has also led to a flurry of interest in other checkpoint inhibitors. The results of the BIRCH4 and POPLAR5 trials of another PD-L1 antibody, atezolizumab, were presented at the European Cancer Congress 2015 in Vienna, Austria. Data from these trials showed that atezolizumab had activity in patients with refractory lung cancer regardless of the number of prior treatments, and that it increased OS in unselected patients with NSCLC compared to single-agent docetaxel. PD-L1 expression levels, both in the cancer cells and in the immune microenvironment, were again positively correlated with treatment benefit.
Studies are also being conducted in which monoclonal antibodies targeting PD-1 or PD-L1 are combined with inhibitors of another immune checkpoint receptor found on T cells, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Although combinations of checkpoint inhibitors are predicted to have greater anti-tumor activity compared to either agent alone, concerns have been raised that patients will be unable to tolerate the overlapping toxicity profiles of these drugs.
Results from a phase 1b trial of the combination of the PD-L1 antibody durvalumab (MEDI 4736) and the CTLA-4 antibody tremelimumab in patients with advanced NSCLC were presented at the European Society for Medical Oncology Asia Congress in Singapore in December 2015, and published this year in The Lancet Oncology.6 The authors identified a dose combination with a manageable safety profile and a response rate of 23%. In contrast to the results from many single-agent PD-1 inhibitor trials, PD-L1 status had no effect on the observed response rates. These results are being followed up with several phase 3 trials, NCT02352948, NCT02453282, and NCT02542293, to examine the efficacy of this combination as first-line therapy for advanced NSCLC in a larger population.
Considering these and other successes for checkpoint inhibitors in thoracic cancer, 2015 signals only the beginning of a gradual move away from chemotherapy to agents that are more effective and safer for the treatment of advanced lung cancer. Clinicians and patients alike have reasons to follow the results of these trials with great interest.✦
References
1. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135.
2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627-1639.
3. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1- positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2015.
4. Besse B, Johnson M, Jänne PA, et al. Phase II, single-arm trial (BIRCH) of atezolizumab as firstline or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC). Paper presented at: European Cancer Congress2015; Vienna, Austria.
5. Vansteenkiste J, Fehrenbacher L, Spira AI, et al. Atezolizumab monotherapy vs docetaxel in 2L/3L non-small cell lung cancer: Primary analyses for efficacy, safety and predictive biomarkers from a randomized phase II study (POPLAR). Paper presented at: European Cancer Congress2015; Vienna, Austria.
6. Antonia S, Goldberg SB, Balmanoukian A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016.