By Mariano Provencio-Pulla, MD, PhD
Posted: December 2018
Patients with stage IIIA NSCLC with clinically evident N2 nodal spread have an overall 5-year survival rate of only 10% to 15%. The aims of therapy in stage III disease are to increase both locoregional and systemic control of the disease. Because distant metastases remain the major reason for treatment failure, it is likely that more effective cytotoxic or other antitumor agents will be required to further improve levels of response and survival.1
Neoadjuvant administration of two doses of nivolumab to patients with early-stage lung cancer has been shown to lead to a major pathologic response—defined as less than 10% of viable tumor cells remaining in the resected specimen—in 45% of tumors.2 Neoadjuvant therapy has theoretical advantages: in vivo assessment of response to chemotherapy helps identify patients who will potentially benefit from adjuvant chemotherapy; early treatment of micrometastatic disease is optimized; a reduction in drug resistance due to early exposure to treatment; and downstaging, with improved resectability, becomes possible.
NADIM Study Details
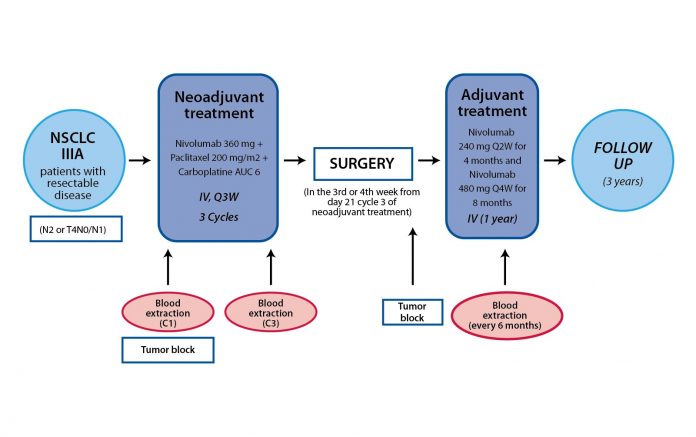
The NADIM Study (CA209-547) was a phase II, single-arm, open-label multicenter study aimed to assess the feasibility, safety, and efficacy of combined neoadjuvant chemotherapy (paclitaxel and carboplatin) and immunotherapy (Fig. 1). The primary endpoint was progression-free survival (PFS) at 24 months from diagnosis, with the data cut-off of June 30, 2018.
The rate of mPR in this study is quite high, particularly in the setting of stage III NSCLC. This potentially bodes well for the future. To this end, a number of phase III trials are comparing combined immunotherapy and chemotherapy to chemotherapy alone in the neoadjuvant setting in resectable NSCLC. Results of these studies should be available in the next 1 to 3 years.
Efficacy results are available for the subset of 30 patients who underwent surgery; no intraoperative complications were documented. Seven of the 30 patients had postsurgical complications, but there was no postoperative mortality.
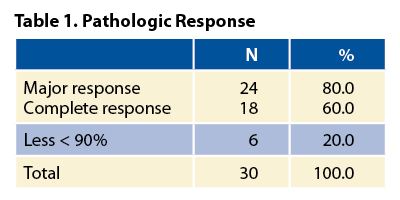
Regarding clinical results, tumor responses after neoadjuvant therapy (100% compliance rate), according to RECIST criteria v1.1 and assessed per CT-SCAN, were as follows: the overall response rate was 70% (21 of 30 patients) and included three complete responses (10%) and 18 partial responses (60%). Stable disease was reported for the remaining nine of 30 patients (30%). Pathologic complete response rates, as shown in Table 1, are unprecedented and highly promising in the context of neoadjuvant therapy of NSCLC. Downstaging occurred in 90% of patients. Toxicity was acceptable. Historically, complete surgical resection,3, 4 tumor downstaging,5 pathologic complete response, and major pathologic responses (mPR)6 have been associated with longterm survival in resectable NSCLC in a retrospective series. The rate of mPR in this study is quite high, particularly in the setting of stage III NSCLC. This potentially bodes well for the future. To this end, a number of phase III trials are comparing combined immunotherapy and chemotherapy to chemotherapy alone in the neoadjuvant setting in resectable NSCLC. Results of these studies should be available in the next 1 to 3 years. Whether mPR is an adequate surrogate for long-term survival in early-stage NSCLC remains to be seen, but these studies should address that issue, and these trials will ultimately determine whether the promise of immunotherapy can be extended to the curative setting. ✦
References:
1. Langer CJ, Leighton JC, Comis RL, et al. Paclitaxel and carboplatin in combination in the treatment of advanced non-small-cell lung cancer (NSCLC): A phase II toxicity, response, and survival analysis (FCCC 93-024). J Clin Oncol. 1995;13(8):1860-1870.
2. Forde PM, Chaft JE, Smith V, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976-1986.
3. Sugarbaker DJ, Herdon J, Kohman LJ, et al. Results of cancer and leukemia group B protocol 8935: A multi-institutional phase II trimodality trial for stage IIIA (N2) non-small-cell lung cancer-Cancer and Leukemia Group B Thoracic Surgery Group. J Thorc Cardiovasc Surg. 1995;109(3):473-485.
4. Kirn DH, Lynch TJ, Mentzer SJ, et al. Multimodality therapy of patients with stage IIIA, N2 non-small–cell lung cancer: Impact of preoperative chemotherapy on resectability and downstaging. J Thorac Cardiovasc Surg. 1993;106(4):696-702.
5. Choi Y S, shim Y M, Kim J, Kim K. Recurrence-free survival and prognostic factors in resected pN2 non-small cell lung cancer. Eur J Cardiothorac Surg. 2002;22(5):695-700.
6. Hellmann M, Chaft JE, William WN, et al. Pathologic response after neoadjuvant chemotherapy in resectable non-small lung cancers: proposal for the use of “major pathologic response” as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42-e50.
7. Martini N, Kris MG, Flehinger BJ, et al. Preoperative chemotherapy for stage IIIa (N2) lung cancer: The Sloan-Kettering experience with 136 patients. Ann Thorc Surg.
8. Martín N. Mediastinal lymph node dissection for lung cancer: The Memorial experience. Chest Surg Clin N Am. 1995;5(2):189-203.