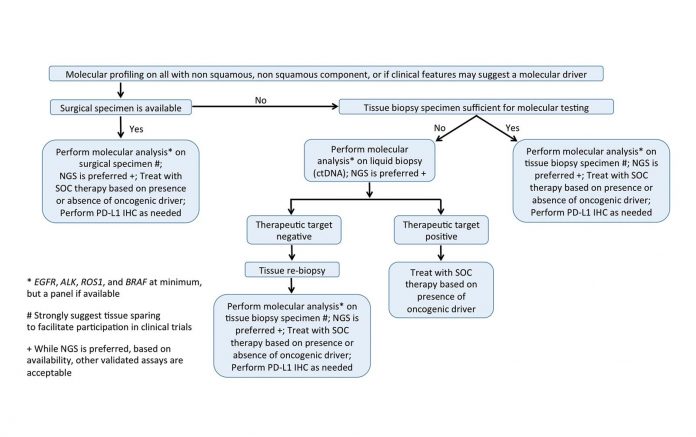
Reproduced with permission. Rolfo C, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. Journal of Thoracic Oncology; available online 6 June 2018. https://doi.org/10.1016/j.jtho.2018.05.030
By Joy Curzio
Posted: August 2018
Liquid biopsy is a powerful tool that can determine a patient’s molecular tumor profile and aid diagnosis, as well as therapeutic choices. Liquid biopsy can be applied as an alternative to tissue testing in cases where tumor testing is not possible or tissue is not adequate for multiple cancer types, including NSCLC. Because of the increasing relevance of this procedure for the optimization of NSCLC clinical management via the identification of predictive biomarkers, either prior to treatment or at progression, the IASLC has issued “The IASLC Statement Paper: Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC),” now available online in the Journal of Thoracic Oncology. The Statement Paper was authored by a multidisciplinary panel of thoracic oncology experts with interest and expertise in liquid biopsy and molecular pathology to evaluate available evidence with the aim of producing a set of recommendations to guide the use of liquid biopsy subsequent molecular analyses.
“It is important to gather experts to collect and interpret the vast amount of information available and to guide best practices ensuring that general oncologists and clinicians have access to the latest and best information in the emerging field of liquid biopsies,” said Fred R. Hirsch, MD, PhD, CEO of the IASLC.
The possibility of using a noninvasive method to understand and identify molecular targets and mechanisms of resistance for current drugs, both targeted agents and immunotherapies, can be extremely beneficial for patients, as will harnessing these strategies to identify new biomarkers. The future of liquid biopsies is undeniably exciting, but there is a need to more clearly understand the latest developments.
“The evolution of liquid biopsies in lung cancer in the past 2 years has been amazing, as evidenced by the U.S. Food and Drug Administration approval for use of circulating tumor DNA (ctDNA) for use with osimertinib in patients with EGFRT790M,” said Christian Rolfo, MD, PhD, MBA, of the University of Maryland Greenebaum Cancer Center and lead author of the statement paper. “The major benefit is the real-time information provided by this noninvasive method. The future also looks very promising for new members of the liquid-biopsy family as exosomes,” Dr. Rolfo noted.
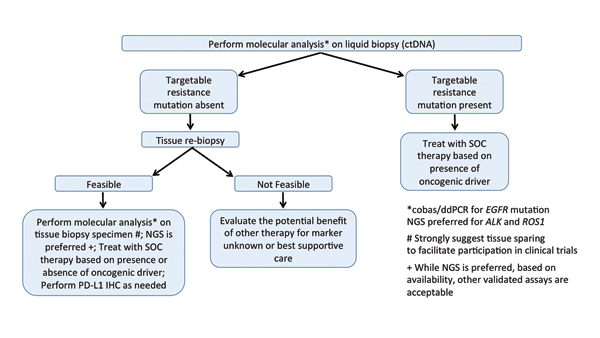
Reproduced with permission. Rolfo C, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. Journal of Thoracic Oncology; available online 6 June 2018. https://doi.org/10.1016/j.jtho.2018.05.030
Given the pace of scientific and therapeutic advances in thoracic oncology, including emerging technologies for liquid biopsy, collecting and distributing up-to-date information is critical for improving outcomes for patients worldwide. The IASLC is committed to serving as a global resource for all involved in lung cancer, the leading cause of cancer-related deaths worldwide.
“This statement is an important tool for clinicians to better understand the latest technologies in liquid biopsies but, more importantly, to answer several frequent questions about use, determination, reporting, and interpretation of the genomic data,” Dr. Rolfo said. “The IASLC is committed to global thoracic oncology education and, with this statement, this goal has been achieved in relation to ctDNA,” Dr. Rolfo concluded. ✦
Related Articles:
Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC










