By Kara Nyberg, PhD
Posted: August 2018
The pace of research focused on new therapies for advanced NSCLC has progressed from a trot to a sprint in recent years. Among immunotherapy options, pembrolizumab emerged as an early frontrunner, along with nivolumab and atezolizumab, in the second-line treatment setting. Pembrolizumab now appears to be surging ahead of other checkpoint inhibitors in the first-line setting based on the collective findings of multiple large phase III KEYNOTE trials.
The latest of these trials reported at the American Society of Clinical Oncology (ASCO) Annual Meeting this past June —KEYNOTE-042—demonstrated that single-agent pembrolizumab significantly improved overall survival (OS) compared with platinum-based chemotherapy in the first-line setting for patients with advanced NSCLC, even those with PD-L1 expression levels as low as 1%.1 How the KEYNOTE-042 findings will translate to clinical practice and whether this heralds the end of frontline chemotherapy for patients with PD-L1–positive disease has yet to be fully determined.
The Past: The KEYNOTE Legacy
KEYNOTE-10 first established the value of pembrolizumab in NSCLC by showing that the PD-1 inhibitor significantly improved OS compared with docetaxel following prior platinum-based chemotherapy, earning it a place as a standard second-line treatment option.2,3
Then came the first-line studies. In KEYNOTE-024, which only allowed patients with PD-L1 expression of 50% or greater (roughly 30% to 35% of all patients with wild-type NSCLC), pembrolizumab monotherapy outperformed platinum-based chemotherapy in the first-line setting for both OS and progression-free survival (PFS).4 Shortly thereafter, pembrolizumab became the first immunotherapy drug approved for the first-line treatment of patients with metastatic NSCLC with high PD-L1 expression.3 More recently, the KEYNOTE-189 and KEYNOTE-407 trials performed in patients with nonsquamous NSCLC and squamous NSCLC, respectively, showed that combining pembrolizumab with standard first-line platinum containing chemotherapy improved OS and PFS compared with chemotherapy alone irrespective of PD-L1 tumor expression. 5,6 These results bolster earlier findings of the phase II KEYNOTE-021 study, which garnered pembrolizumab accelerated approval for use in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous NSCLC.7
The KEYNOTE-042 trial demonstrated that single-agent pembrolizumab significantly improved overall survival compared with platinum-based chemotherapy in the first-line setting for patients with advanced NSCLC.
In light of this work, the next logical step was to see if survival could be extended and the toxicity of chemotherapy averted by leveraging pembrolizumab alone in patients with lower levels of PD-L1 expression (1% to 49%). Hence the rationale for the KEYNOTE-042 trial.
The Present: KEYNOTE-042
Lead investigator Gilberto Lopes, MD, MBA, of the University of Miami Sylvester Comprehensive Cancer Center, presented the KEYNOTE-042 findings at the ASCO Annual Meeting during the main plenary session. This randomized, open-label, phase III trial included patients with locally advanced or metastatic NSCLC of squamous or nonsquamous histology with PD-L1–positive expression but without sensitizing EGFR mutations or ALK alterations.
Like other pembrolizumab trials that came before it, PD-L1 tumor expression was assessed using the 22C3 immunohistochemistry assay. The investigators employed the tumor proportion score (TPS) for patient stratification and analysis. A PD-L1 score of ≥ 1% was mandatory for entry into the trial.
Researchers stratified the 1,274 patients included in KEYNOTE-042 by region (East Asia vs. other regions), ECOG performance status (0 vs. 1), histology (nonsquamous vs. squamous), and PD-L1 TPS (1% to 49% vs. ≥ 50%). Patients were randomly assigned to pembrolizumab or chemotherapy, consisting of up to six cycles of paclitaxel and carboplatin or pemetrexed and carboplatin with optional pemetrexed maintenance (nonsquamous histology only) at the investigator’s discretion. OS comprised the primary endpoint of interest, which was tested sequentially for those with a PD-L1 TPS of ≥ 50%, ≥ 20%, and ≥ 1%— the latter representing the entire study population.
Patient baseline characteristics were well balanced across the two treatment arms. In both groups, participants had a median age of 63 years, 71% were men, 29% were enrolled in East Asia, 39% had squamous histology, 47% had a PD-L1 TPS of ≥ 50%, and 78% were current or former smokers.
Pembrolizumab monotherapy excelled over chemotherapy across all TPS subgroups, with greater benefits seen with higher PD-L1 expression. Among patients with PD-L1 TPS of ≥ 50%, median OS reached 20 months with pembrolizumab versus 12.2 months with chemotherapy (hazard ratio [HR] 0.69, 95% CI [0.56, 0.85]; p = 0.0003). In the PD-L1 TPS ≥ 20% subgroup, median OS was 17.7 months with pembrolizumab vs. 13.0 months with chemotherapy (HR 0.77, 95% CI [0.64, 0.92]; p = 0.0020). Finally, among patients with PD-L1 TPS of ≥ 1%, comprising the entire study population, median OS reached 16.7 months with pembrolizumab versus 12.1 months with chemotherapy (HR 0.81, 95% CI [0.71, 0.93]; p = 0.0018).
PFS, the secondary endpoint of the trial, was not met. Although pembrolizumab improved median PFS in comparison with chemotherapy in patients with PD-L1 TPS of ≥ 50% (7.1 vs. 6.4 months; HR 0.81, 95% CI [0.67, 0.99]), the p value of 0.0170 did not meet the protocol-specified significance boundary. No significant PFS differences were observed between arms for patients with PD-L1 TPS of ≥ 20% and the entire cohort with PD-L1 TPS of ≥ 1%.
Despite a longer duration of treatment exposure, grades 3 to 5 treatment-related adverse events occurred much less often with pembrolizumab than with chemotherapy (17.8% vs. 41.0%). However, as expected, grades 3 to 5 immune-related adverse events and infusion reactions occurred more frequently among patients treated with pembrolizumab versus chemotherapy (8.0% vs. 1.5%). The respective rates of treatment discontinuation (9.0% vs. 9.4%) and treatment-related deaths (2.0% vs. 2.3%) proved to be comparable between the arms.
“These data, therefore, confirm and extend the role of pembrolizumab monotherapy as a standard first-line treatment for patients with PD-L1-expressing tumors,” Dr. Lopes concluded. “This better safety and activity profile of pembrolizumab suggests that it is an appropriate treatment for patients at any level of PD-L1 positivity.”
The Future: Pembrolizumab for All?
Given the totality of the KEYNOTE findings, the key question emerging is not whether pembrolizumab should be used in treatment-naive patients with advanced PD-L1–positive disease that lacks driver mutations—that seems to be a given at this point. Rather, the critical question is whether pembrolizumab should be used alone or in combination with chemotherapy. “Is PD-L1 monotherapy really the answer for everyone with PD-L1 of 1% or higher?” posed Leena Gandhi, MD, PhD, of the New York University Perlmutter Cancer Center, who critiqued the KEYNOTE-042 findings following Dr. Lopes’ presentation of the results.
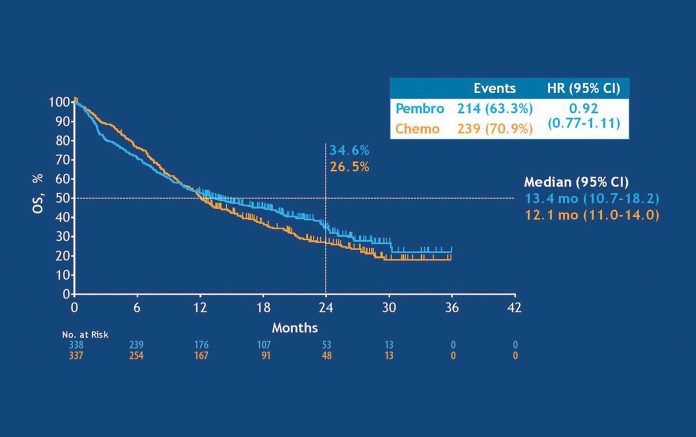
In answer to this question, Dr. Gandhi referred to an exploratory analysis that Dr. Lopes showed during his KEYNOTE-042 presentation in which little survival advantage emerged with pembrolizumab versus chemotherapy in patients with a TPS of 1% to 49% (median OS: 13.4 vs. 12.1 months; HR 0.92, 95% CI [0.77, 1.11]). She argued that the OS benefit associated with pembrolizumab in KEYNOTE-042 is driven by the high PD-L1 subgroup (TPS ≥ 50%); the benefits are not as clear-cut for those with PD-L1 TPS of 1% to 49%.
Because of this, Dr. Gandhi still foresees an important role for frontline chemotherapy in selected patients with PD-L1–positive disease. “Patients with low or no PD-L1 expression likely should get some type of combination therapy,” she said. Dr. Gandhi drew on data from KEYNOTE-189 and KEYNOTE-407 and emphasized that these two frontline trials documented clear, consistent improvements in OS, PFS, and response rates with first-line pembrolizumab and platinum containing chemotherapy over chemotherapy alone in all patients, regardless of PD-L1 expression, which cannot be said for KEYNOTE-042.
All told, consensus seems to be emerging that in the absence of driver mutations, patients with PD-L1 expression of ≥ 50% should receive first-line pembrolizumab monotherapy, whereas those with lower expression levels would do best with a pembrolizumab-chemotherapy combination. However, these are not hard-and-fast rules. Patient-specific factors, such as high tumor burden or poor performance status, may prompt the decision to add or eliminate chemotherapy, as necessary, to optimize outcomes for each individual patient. ✦
EDITOR’S NOTE
Although the OS data are striking, there is no apparent benefit for those patients with PD-L1 TPS of < 50%. In this sense, KEYNOTE-042 confirms KEYNOTE-024, but it does not really alter the therapeutic landscape, although the results are likely to garner pembrolizumab expanded approval to patients with any degree of PD-L1 expression.
–Corey J. Langer, MD, Editor
References:
1. Lopes G, Wu Y, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/ metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. Paper presented at: American Society of Clinical Oncology Annual Meeting; June 1-5, 2018; Chicago, IL.
2. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1- positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
3. Pai-Scherf L, Blumenthal GM, Li H, et al. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. 2017;22(11):1392-1399.
4. Reck M, Rodríguez-Abreu D1, Robinson AG1, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
5. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092.
6. Paz-Ares L, Luft A, Tafreshi A, et al. KEYNOTE-407: phase 3 study of carboplatinpaclitaxel/nab-paclitaxel with or without pembrolizumab for metastatic squamous NSCLC. Paper presented at: American Society of Clinical Oncology Annual Meeting; June 1-5, 2018; Chicago, IL.
7. US Food and Drug Administration. Pembrolizumab (Keytruda) 5-10-2017. https://www.fda.gov/drugs/informationondrugs/ approveddrugs/ucm558048.htm. Last updated May 11, 2017. Accessed July 11, 2018.