By Kara Nyberg, PhD
Posted: August 2018
Treatment advances for metastatic squamous cell carcinoma have long lagged behind those for other NSCLC subtypes, forcing patients with this difficult-to-treat disease to settle with platinum-based chemotherapy as the best treatment option. But the tide now appears to be turning. As presented at the American Society of Clinical Oncology (ASCO) meeting in Chicago this past June, two dedicated squamous cell carcinoma studies— KEYNOTE-407 and IMpower131—have shown that immunotherapy–chemotherapy combinations can improve outcomes over chemotherapy alone.1,2 Importantly, KEYNOTE-407 provides irrefutable evidence that such combinations can significantly improve patient survival regardless of PD-L1 status, ushering in a new standard of care for squamous disease.
KEYNOTE-407: New Standard
The global phase III KEYNOTE-407 trial included 559 patients with squamous NSCLC without regard to tumor PD-L1 expression levels.1 Individuals were randomly assigned to receive four cycles of carboplatin and paclitaxel or nabpaclitaxel, plus either pembrolizumab or placebo. Maintenance pembrolizumab or placebo was administered in accord with patients’ initial treatment assignment. Placebo-treated patients could cross over to receive pembrolizumab monotherapy upon disease progression.
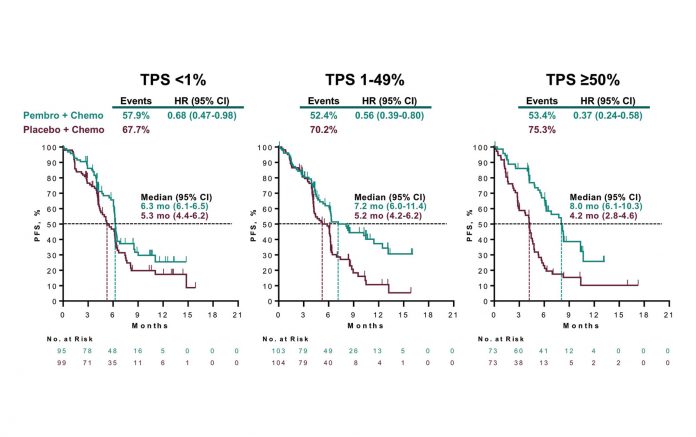
During a Clinical Science Symposium, KEYNOTE-407 investigator Luis G. Paz- Ares, MD, PhD, reported the results of the second interim analysis, which reflected sufficient events to gauge both overall survival (OS) and progression-free survival (PFS), the co-primary endpoints of the trial. The data showed that combining pembrolizumab with conventional chemotherapy in the first-line setting significantly prolonged median OS to 15.9 months compared with 11.3 months with chemotherapy alone (hazard ratio [HR] 0.64, 95% CI [0.49, 0.85]; p = 0.0008). Moreover, the OS benefit observed with the pembrolizumab-chemotherapy regimen persisted across all relevant patient subgroups, including those with tumor PD-L1 expression categorized as low (<1%; HR 0.61, 95% CI [0.38, 0.98]), intermediate (1%-49%; HR 0.57, 95% CI [0.36, 0.90]), and high (≥50%; HR 0.64, 95% CI [0.37, 1.10]).
The pembrolizumab-chemotherapy combination also proved superior to chemotherapy alone with regard to median PFS (6.4 vs. 4.8 months; HR 0.56, 95% CI [0.45, 0.70]; p < 0.0001), objective response rate (58.4% vs. 35.0% at the first interim analysis; p = 0.0004), and the median duration of response (7.7 vs 4.8 months).
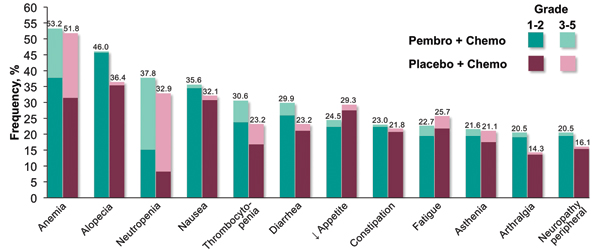
Adverse events occurred at similar frequencies in the pembrolizumab–chemotherapy and chemotherapy-alone arms. However, immune-mediated adverse events and infusion reactions occurred more often with the addition of pembrolizumab to chemotherapy, as compared to without, both overall (28.8% vs. 8.6%) and for grades 3 to 5 adverse events (10.8% vs. 3.2%). The most common immune-mediated adverse events associated with the pembrolizumab arm included hypothyroidism (7.9%), hyperthyroidism (7.2%), and pneumonitis (6.5%).
“These data suggest pembrolizumab plus carboplatin and paclitaxel or nabpaclitaxel should become a new standard of care for the first-line treatment of metastatic squamous NSCLC across all levels of PD-L1 expression,” concluded Dr. Paz-Ares. Charles G. Drake, MD, PhD, who critiqued the KEYNOTE-407 results following Dr. Paz-Ares’ presentation, agreed. “This trial is clearly a win,” he said.
The KEYNOTE-407 results complement those of the momentous KEYNOTE-189 trial,3 which demonstrated that adding pembrolizumab to first-line chemotherapy significantly improved median OS regardless of PD-L1 tumor expression in patients with metastatic nonsquamous NSCLC.
IMpower131: Atezolizumab with Chemotherapy Still Under Study
Whether atezolizumab and chemotherapy might represent another new frontline standard of care for patients with metastatic squamous NSCLC remains to be determined. Robert M. Jotte, MD, PhD, presented interim findings of the randomized phase III IMpower131 trial, in which 1,021 patients were randomly assigned to treatment with atezolizumab plus carboplatin/ paclitaxel, atezolizumab plus carboplatin/ nab-paclitaxel, or carboplatin/ nab-paclitaxel (the control arm).2 Patients received 4 or 6 cycles of chemotherapy with or without atezolizumab, followed thereafter by atezolizumab maintenance therapy or best supportive care.
Median PFS, one of the two co-primary endpoints, reached 6.3 months with atezolizumab plus carboplatin/nab-paclitaxel compared with 5.6 months for carboplatin/ nab-paclitaxel alone (HR 0.71, 95% CI [0.60, 0.85]; p = 0.0001). The PFS rate at 1 year, which reflects additional separation of the Kaplan-Meier curves beyond the median values, was 24.7% for atezolizumab plus carboplatin/nabpaclitaxel and 12.0% for carboplatin/ nab-paclitaxel alone. Among PD-L1– expressing subgroups, the PFS difference between these two respective regimens was greatest in the subgroup of patients with the highest level of PD-L1 expression (median PFS: 10.1 vs. 5.5 months; HR 0.44, 95% CI [0.27, 0.71]).
However, an initial look at median OS, the other co-primary endpoint, failed to show a difference in outcome between the control arm and the combination of atezolizumab and carboplatin/nabpaclitaxel in the intent-to-treat population (14.0 vs. 13.9 months). In the PD-L1 subgroups, median OS trended favorably for the atezolizumab-containing arm in the high PD-L1–expressing subgroup (23.6 vs. 14.1 months; HR 0.56, 95% CI [0.32, 0.99]) but—unexpectedly—unfavorably in the low PD-L1–expressing subgroup (12.4 vs. 16.6 months; HR 1.34, 95% CI [0.95, 1.90]).
“A new combination therapy needs to show an OS benefit before it’s adopted as a standard of care,” commented Tom Stinchcombe, MD, who discussed the IMpower131 findings. Longer follow-up is needed to see if an OS difference emerges over time that might favor the atezolizumab-containing combination. ✦
References:
1. Paz-Ares L, Luft A, Tafreshi A, et al. KEYNOTE-407: phase 3 study of carboplatinpaclitaxel/nab-paclitaxel with or without pembrolizumab for metastatic squamous NSCLC. Paper presented at: American Society of Clinical Oncology Annual Meeting; June 1-5, 2018; Chicago, IL.
2. Jotte R, Cappuzzo F, Vynnychenko I, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. Paper presented at: American Society of Clinical Oncology Annual Meeting; June 1-5, 2018; Chicago, IL.
3. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092
IMpower130: Atezolizumab Shows OS, PFS Benefit
According to results of the phase III IMpower130 study (NCT02367781), the addition of atezolizumab to carboplatin and nab-paclitaxel in the first-line setting for patients with advanced nonsquamous NSCLC was found to increase overall and progression-free survival rates compared with chemotherapy alone. IMpower130 is multicenter, openlabel, randomized study in which 724 patients were randomized (2:1) to receive either atezolizumab plus carboplatin and nab-paclitaxel (Arm A) or carboplatin and nab-paclitaxel alone (Arm B, control). The coprimary endpoints were overall survival (OS) and progression-free survival (PFS) in the intent-to-treat wild-type population. No new safety signals were reported at the data cutoff. Formal results will be presented at an upcoming meeting. In addition, the U.S. Food and Drug Administration (FDA) recently accepted a supplemental Biologics License Application (sBLA) and granted Priority Review for atezolizumab, in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of people with metastatic nonsquamous NSCLC based on both a PFS and OS benefit seen in IMpower 150, in comparison to standard chemotherapy and bevacizumab. The FDA is expected to make a final decision regarding approval in early September 2018. (Read the AACR recap for details on IMpower150.)