New Lung Cancer Screening Guidelines Will Save Lives
Reaction was swift and positive last week to new draft guidelines from the U.S. Preventive Services Task Force lowering the requirements for low dose CT imaging as a screening tool for those at risk for lung cancer. Current recommendations call for low-dose CT scans for 55- to 80-year-olds who have smoked at least 30 pack-years over a lifetime, and either still smoke or have quit within the past 15 years.
The new draft guidelines lower those recommendations to 50 years of age and to 20 pack-years of smoking cigarettes.
“Extending lung cancer screening to those aged 50 and older versus 55 and older, as well as to those with 20 pack-years smoking history versus 30, will save more lives and include high-risk individuals who would otherwise be excluded from screening altogether,” said Dr. Chandra Belani, Chief Science Officer at the International Association for the Study of Lung Cancer.
For Dr. Jacob Sands, a thoracic oncologist at Dana-Farber Cancer Institute and instructor of Medicine at Harvard Medical School, the new guidelines will have a significant impact on access to screening. Sands is also a member of the IASLC Screening and Early Detection Committee.
“This increase in screening eligibility is a big deal as it doubles the number of people eligible for lung screening, which will increase the number of lives saved from early detection of lung cancer. This has the potential to decrease disparity, as more African Americans and women with high-risk of developing deadly lung cancer will be eligible for lung screening. Although the reduction in age and decrease in pack-years is a substantial improvement, the limitation to only current smokers or those that quit within the past 15 years presents a barrier, including individuals with additional risk factors,” he said.
The COVID-19 pandemic adds a unique sense of urgency to lung cancer screening and detection, according to Dr. Matthew Steliga, chair of the IASLC Tobacco Control and Smoking Cessation Committee and surgical oncologist at Winthrop P. Rockefeller Cancer Institute in Little Rock, Ark. Dr. Steliga bylined an article in the IASLC Lung Cancer News (ILCN) about the serious risk of tobacco and lung cancer.
“As the pandemic has progressed, we have learned about risk factors for poorer outcomes. Those with advanced age, chronic lung or heart disease, diabetes, or obesity are at increased risk of complications and death from COVID-19. Chemotherapy, immunosuppressant medications, or immunologic diseases could potentially lead to increased risk from infection. Our patients with cancer—particularly those with lung cancer—may be a particularly vulnerable group. Advanced age, medical comorbidities (particularly respiratory disease), and altered immune function may be a perfect storm of risk. Although most factors cannot be altered, there is one particular alterable risk that may often be overlooked by patients and physicians: smoking or vaping,” Dr. Steliga wrote.
The IASLC journal, the Journal of Thoracic Oncology, has published several studies supporting low-dose CT for lung cancer screening. One of the original studies demonstrating the effectiveness of CT screening for lung cancer, The NELSON Trial, was first presented at the IASLC 19th World Conference on Lung Cancer in 2018 in Toronto in 2018. ✦
Interim Results for CheckMate-743 Positive for Combination Nivolumab/Ipilimumab in Previously Untreated MPM
April 20, 2020—Based on a pre-specified interim analysis conducted by an independent Data Monitoring Committee, the phase III CheckMate-743 trial found a statistically significant and clinically meaningful improvement in OS for nivolumab plus ipilimumab compared with chemotherapy (pemetrexed and either cisplatin or carboplatin) for patients with previously untreated unresectable malignant pleural mesothelioma (MPM). Formal data have yet to be released, but the press release stresses that no new safety signals were observed.
Phase III interim results were based on 606 total participants with unresectable MPM who were randomly assigned to nivolumab plus ipilimumab (3 mg/kg nivolumab every 2 weeks; 1 mg/kg ipilimumab every 6 weeks) or to chemotherapy.
Secondary endpoints included ORR, disease control rate, PFS, and efficacy measures based on PD-L1 expression level. ✦
Two New Drug Approvals for Mutation-Driven Cancers Change the Th erapeutic Landscape of NSCLC
May 2020—During the first full week of May, The US Food and Drug Administration (FDA) approved two separate targeted therapies for RET and MET-driven lung cancers, neither of which had pre-existing marker-specific approvals.
Selpercatinib for RET-driven NSCLC
Based on the phase I/II LIBRETTO-001 trial, the largest trial of patients with RETdriven cancers, selpercatinib received accelerated approval on May 9, 2020 for patients with metastatic RET fusion-positive NSCLC, agnostic to line of therapy.
LIBRETTO-001 enrolled both treatment-naive (n = 39) and heavily pretreated (n = 105) patients with a RET fusion-positive NSCLC. The ORR was 85% (95% CI: 70%, 94%) and 64% (95% CI: 54%, 73%), respectively. The median DoR was not reached for treatment-naive patients, but the minimum DoR was reported as 12 months; median DoR for pretreated patients was 17.5 months (95% CI: 12, not reported).
Prespecified secondary endpoints included central nervous system (CNS) ORR and CNS DoR. Among those patients with NSCLC and measurable brain metastases, 10 of 11 demonstrated intracranial responses. Of these, 100% demonstrated a CNS DoR of > 6 months.
The most frequent serious adverse event was pneumonia. There was a 5% discontinuation rate for patients in LIBRETT0-001, across all cancer types.
Capmatinib for MET-driven NSCLC
Just 3 days prior to selpercatinib’s approval, accelerated approval was granted to capmatinib for patients with MET exon 14 skipping mutation-driven NSCLC. A companion diagnostic—the FoundationOne CDx assay— was also approved.
Efficacy was demonstrated in the multicenter, nonrandomized, open-label, multicohort GEOMETRY mono-1 trial, which enrolled 97 patients with metastatic NSCLC with confirmed MET exon 14 skipping mutation. Capmatinib (400 mg, oral, twice daily) was administered until disease progression or unacceptable toxicity. ORR was evaluated by a blinded independent review committee using Response Evaluation Criteria in Solid Tumors, version 1.1.
ORR for treatment-naive patients (n = 28) was 68% (95% CI; 48%, 84%) with a median response duration of 12.6 months (95% CI: 5.5, 25.3). ORR was 41% (95% CI: 29%, 53%) for patients who had received prior therapy (n = 69) with a median response duration of 9.7 months (95% CI: 5.5, 13.0).
The most common adverse reactions (in ≥ 20% of patients) were peripheral edema, nausea, fatigue, vomiting, dyspnea, and decreased appetite. Sensitivity to sunlight is possible so patients should be advised to take precautions including covering their skin and using sunscreen. ✦
FDA Approves Durvalumab for Extensive-Stage SCLC
March 27, 2020—The U.S. Food and Drug Administration approved durvalumab in combination with etoposide and either carboplatin or cisplatin for the first-line treatment of patients with extensive-stage SCLC. The approval was based on the randomized, multicenter, open-label, active-controlled CASPIAN trial, which compared durvalumab plus chemotherapy vs chemotherapy alone for previously untreated patients with extensive-stage SCLC.
In CASPIAN, median OS was 13.0 months (95% CI: 11.5, 14.8) for the combination compared with 10.3 months (95% CI: 9.3, 11.2) for chemotherapy alone (HR 0.73; 95% CI: 0.59, 0.91; p = 0.0047). Median PFS was 5.1 months (95% CI: 4.7, 6.2) and 5.4 months (95% CI: 4.8, 6.2), respectively. ORR was 68% (95% CI: 62, 73) and 58% (95% CI: 52, 63), respectively.
With this approval, durvalumab joins atezolizumab in combination with standard platinum and etoposide as options for front-line treatment in the management of chemotherapy-naive extensive-stage SCLC. ✦
Tell the Trump Administration That Vape Shops Are Not “Essential Businesses”
Join the Campaign for Tobacco-Free Kids in signing a petition to close vape shops during the pandemic.
Sharp Decline of Cancer Death Rates Partially Due to Lung Cancer Advances
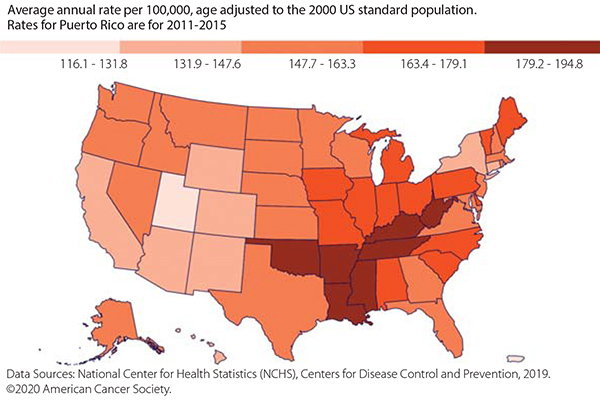
In early January 2020, the American Cancer Society (ACS) released a statement noting that the cancer mortality rate had dropped 2.2% between 2016 and 2017. This was the most notable decline ever recorded and, according to an NPR interview with Rebecca Siegel, MPH, scientific director for surveillance research at the ACS, “It seems to be driven by accelerating declines in lung cancer mortality.” New therapies could have contributed, along with declining smoking rates. However, it is yet to be determined how the dramatic increase in vaping rates, especially among youth, will offset the decline in smoking rates. Long-term risks for vaping also need to be parsed out from the more immediate risks. ✦
Updated Guidelines for Stage IV, Non-Driver NSCLC
Updated recommendations for systemic therapy for patients with stage IV NSCLC and no EGFR or ALK mutations were issued in late January 2020 by the American Society of Clinical Oncology and Ontario Health (Cancer Care Ontario) NSCLC Expert Panel. The updates were based on a systematic review of randomized controlled trials from December 2015 to 2019. The updated guideline is available on ASCO.org or in the Journal of Clinical Oncology. Recommendations for treatment of patients with EGFR/ALK mutations will be issued in the future. ✦
Approval Expected for Ramucirumab/Erlotinib Combination for NSCLC
On February 26, 2020, the Oncologic Drugs Advisory Committee, a U.S. Food and Drug Administration (FDA) advisory committee, voted to recommend approval of combination ramucirumab injection and erlotinib for firstline therapy for patients with NSCLC and EGFR exon 19 deletions or exon 21 substitution mutations and NSCLC based on data from the phase III RELAY trial. The narrow vote of 6-5 reflected concern regarding lack of proven OS improvement while acknowledging the significant PFS benefit. Also, some concern was expressed regarding toxicity and the every-2-week infusion schedule.
In the RELAY trial, 449 patients with metastatic NSCLC and EGFR exon 19 deletions or exon 21 L858R mutations who had not received prior treatment were randomly assigned to 150 mg of daily erlotinib plus either 10 mg/kg of ramucirumab or placebo every 2 weeks. The primary endpoint of investigatorassessed PFS proved superior for the full combination-therapy group (median 19.4 months) than the erlotinib/placebo group (12.4 months; HR=0.59; 95% CI, 0.46-0.76). Median PFS benefit was very similar regardless of mutation: 19.6 months for patients with exon 19 deletions and 19.4 months for exon 21 L858R mutations.
There were 79 deaths at the time of data cutoff in January 2019, at which time the hazard ratio (HR) for OS was 0.83 (95% CI, 0.53-1.3). After reviewing updated survival data (as of December 31, 2019) in response to a request from the FDA, researchers reported 125 deaths and an HR for OS of 0.92 (95% CI, 0.65-1.32).
Grade-3 or higher adverse events were experienced more often by patients who received the combination therapy (72%) compared with those who received erlotinib/placebo (54%). Th e most commonly reported adverse event in the combinationtherapy group was hypertension. One treatment-emergent adverse event, also in the combination-therapy group, resulted in death. ✦
FDA Issues Two Priority Reviews
On February 17, 2020, the US Food and Drug Administration (FDA) granted a priority review designation for lurbinectedin’s new drug application (NDA). The NDA is for treatment of patients with SCLC and progression after a platinum-based therapy and was based on data presented at the 2019 American Society of Clinical Oncology Annual Meeting.
A Prescription Drug User Free Act (PDUFA) target action date has been set as August 16, 2020.
Earlier in the year, the FDA granted priority review to the supplemental biologics license application for nivolumab and ipilimumab for firstline treatment of metastatic or recurrent NSCLC for patients with wildtype EGFR or ALK.
The application was partially based on data from CheckMate-227. The PDUFA target action date is May 15, 2020. ✦
New First-Line Treatment for Metastatic Nonsquamous NSCLC
December 4, 2019—Indications for atezolizumab have been expanded to include its use in combination with chemotherapy for the first-line treatment of adults with EGFR/ALK wildtype metastatic nonsquamous NSCLC.
This approval was based on data from the phase III IMpower130 study in the intention-to-treat wild-type population, which demonstrated an improved median OS for atezolizumab plus chemotherapy vs chemotherapy alone (18.6 vs 13.9 months, respectively; HR = 0.80; 95% CI = 0.64–0.99; p = 0.04). Progression-free survival and risk of death were also improved (median PFS = 7.2 vs 6.5 months; HR = 0.75; 95% CI = 0.63–0.91; p = 0.002). No new safety signals for the agent were identified in the trial, with 73.2% of patients who received the agent experiencing grade 3/4 adverse events compared with 60.0% of patients who received chemotherapy alone.
Atezolizumab is already approved as first-line therapy for metastatic nonsquamous NSCLC in combination with bevacizumab, paclitaxel, and carboplatin. It is also approved as a first-line therapy for patients with extensive-stage SCLC when combined with chemotherapy.
Osimertinib Performs as Expected for Previously Untreated Patients with NSCLC
September 29, 2019—The final OS results from an analysis conducted from the phase III FLAURA trial demonstrated significantly improved OS for osimertinib compared with gefitinib or erlotinib for previously untreated patients with locally advanced or metastatic NSCLC and with EGFR exon 19 or L858R mutations. Median OS for osimertinib was 38.6 months vs 31.8 months for erlotinib or gefitinib (HR 0.799; 95.05% CI, 0.641-0.997; p = 0.0462), even with crossover allowed for first-generation– treated patients who developed the T790M resistance mutation. OS rates for osimertinib at 1, 2, and 3 years were 89%, 74%, and 54% vs 83%, 59%, and 44%, respectively. Overall, the median follow-up for patients who received osimertinib was 35.8 months vs 27.0 for the comparator, during which time 11 fewer deaths occurred in the osimertinib arm.
FLAURA discussant Pasi A. Jänne, MD, said in a press statement that the magnitude of benefit varied by subgroup, although outcomes were consistent across groups. “The study findings are practice changing; however, osimertinib is already approved for acquired resistance due to EGFR T790M in 87 countries worldwide and furthermore, it is approved for first-line treatment in 78 countries worldwide. But barriers to first-line use are cost and/or lack of reimbursement,” said Dr. Jänne.
CheckMate-227: Nivolumab/Ipilimumab Superior to Chemo in First-Line NSCLC with PD-L1 Expression > 1%
September 28, 2019—Part 1a of the phase III CheckMate-227 trial was successfully completed, with nivolumab plus low-dose ipilimumab yielding superior OS (median 17.1 months) compared to chemotherapy (14.9 months) for patients with NSCLC and PD-L1 expression of > 1% (HR 0.79; 97.72% CI: 0.65-0.96; p = 0.007). OS was also improved for patients with PD-L1 < 1% who received the combination therapy and in the study population overall. The safety profile for the combination was similar to previous findings published in The New England Journal of Medicine.1
A prior report of this study1 showed superior overall response and PFS for ipilimumab/nivolumab in patients with high TMB compared to standard platinum- based chemotherapy, independent of PD-L1 status, but high TMB did not translate into an OS benefit.
Reference:
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378(22):2093-2104.
Atezolizumab Monotherapy Improves OS in Frontline NSCLC with High PD-L1
September 27, 2019—Atezolizumab monotherapy improved OS compared with platinum-based chemotherapy for previously untreated patients with advanced NSCLC and high PD-L1 expression (> 50% on tumor cells or > 10% on tumor-infiltrating immune cells), regardless of histology. The OS findings were confined to ALK/EGFR wild- type tumors. The phase III IMpower110 trial accrued 572 treatment-naive patients and reported on 555 wild type (ALK/ EGFR mutation negative) with previously untreated advanced nonsquamous or squamous NSCLC. Patients were randomly assigned to single-agent therapy with atezolizumab or to platinum-based chemotherapy. The primary endpoint was OS by PD-L1 subgroup; secondary endpoints included PFS, ORR, and duration of response. Median OS at 15.7 months follow-up (range 0-35) was 20.2 months for patients who received atezolizumab vs 13.1 months for those who received chemotherapy (HR = 0.59; 95% CI: 0.40-0.89; p = 0.0106). PFS was also improved with atezolizumab, with a median PFS of 8.1 months vs. 5.0 months (HR = 0.63; 95% CI: 0.45- 0.88; p = 0.007) in the same population. The ORR was 38.3% vs. 28.6%, respectively, and the median DOR was not reached for atezolizumab (vs 6.7 months for chemotherapy).
The Death of MERU
August 29, 2019—AbbVie closed the phase III placebo-controlled MERU trial evaluating rovalpituzumab-tesirine, an antibody–drug conjugate targeting the delta-like ligand 3 protein, as first-line maintenance therapy for extensive-stage SCLC in patients with stable disease or response to first-line etoposide and platinum. No survival benefit was seen versus placebo at a pre-planned interim analysis. The MERU data will be published in the future.
Entrectinib Wins Approval for ROS1-Positive NSCLC
August 15, 2019—The U.S. Food and Drug Administration (FDA) granted approval for entrectinib for the treatment of adults with ROS1-positive metastatic NSCLC and accelerated approval for treatment of both pediatric patients older than 12 and adults with solid tumors that harbor an NTRK fusion. The latter indication is tumor-agnostic, though it should be noted the NTRK fusions generally occur in fewer than 1% of patients with advanced NSCLC.
The approvals were based on data from the phase II STARTRK-2, phase I STARTRK-1, and the phase I ALKA-372-001 trials, which included 51 patients with ROS1-positive metastatic NSCLC. Various doses and schedules were examined in the three trials, but 90% of patients received 600 mg of oral entrectinib once daily, the recommended dose. Pooled data from these ongoing trials showed a 78% ORR (95% CI: 65, 89); 55% achieved responses for 12 months or more (median, 24.6 months; 1.8-36.8 months).
The most serious adverse reactions regardless of causality, included congestive heart failure, CNS effects, skeletal fractures, hepatotoxicity, hyperuricemia, QT interval prolongation, and vision disorders. The most common adverse reactions in at least 20% of patients included, but were not limited to, fatigue, constipation, dysgeusia, edema, dizziness, and diarrhea.
CASPIAN Trial to Report Improved OS for Durvalumab in SCLC
June 27, 2019—According to an interim analysis, the phase III CASPIAN trial (NCT03043872) of durvalumab plus etoposide and chemotherapy for previously untreated late-stage SCLC has met its primary endpoint of clinical and statistical OS improvement for durvalumab. Safety and tolerability was in line with known data for this drug.
This triple-arm, open-label, multicenter, global, randomized phase III trial was conducted in more than 200 centers in 22 countries. Eligible patients received standard etoposide/ platinum-based chemotherapy, either alone or in combination with durvalumab or durvalumab and tremelimumab.
Data will be presented at the 2019 IASLC World Conference on Lung Cancer.
Pembrolizumab Receives Third-Line Approval for Metastatic SCLC
June 17, 2019—Pembrolizumab received accelerated approval from the U.S. Food and Drug Administration for treatment of patients with metastatic SCLC with disease progression during or after platinum-based chemotherapy and at least one other line of therapy.
Approval was based on results from KEYNOTE-158 and KEYNOTE-028. A total of 83 patients received either 200 mg of IV pembrolizumab every 3 weeks (64 patients), which was found to be the recommended dosage, or 10 mg/kg IV every 2 weeks (19 patients). Treatment continued for a maximum of 24 months or until disease progression or unacceptable toxicity.
The ORR was 19% (95% CI: 11-29), and the CR rate was 2%. For the 16 patients who demonstrated a response, the percentage with durable responses at 6, 12 or more, and 18 or more months were 94%, 63%, and 56%, respectively. Study treatment was discontinued due to adverse events (AEs) in 9% of patients; 25% had at least one dose withheld due to an AE. Common AEs were fatigue, decreased appetite, cough, nausea, and constipation, each of which was in at least 20% of patients. Serious AEs occurred in 31% of patients, with pneumonia and pleural effusion being the most frequent (> 2%).
Pembrolizumab was granted orphan drug designation for SCLC in October 2017.
A Drug for an Undruggable Target
June 3, 2019—A novel small-molecule inhibitor targeting KRAS, known as AMG 510, demonstrated a 50% response rate in patients with NSCLC who had KRASG12C mutations, which are found in approximately 13% of lung adenocarcinomas and up to 3% of other solid tumors. Initial safety and response data from a first-in-human, open-label, phase I trial of this novel small-molecule KRAS inhibitor were presented at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting (NCT03600883) and found the agent to be well tolerated.
All patients in phase I had a KRASG12C mutation confirmed by DNA sequencing. Eligible patients had measurable or evaluable disease, an ECOG PS < 2, and a life expectancy of > 3 months. Patients with brain metastases and myocardial infarction within 6 months were excluded.
In the initial cohort of six patients with NSCLC, 15 with colorectal cancer, and one other, 10 discrete grade 1 or 2 treatment-related adverse events (TRAEs) were reported in five patients; there were no dose-limiting toxicities. In an update at ASCO, of 10 patients with advanced NSCLC and KRASG12C mutations, five registered a partial response. Twenty patients of the initial 22 are continuing treatment; maximum-tolerated dose has yet to be established.
Device Becomes Second Approved Therapy for Unresectable MPM
May 23, 2019—A new device was approved by the U.S. Food and Drug Administration (FDA) for use in the first-line setting for treatment of unresectable, locally advanced or metastatic malignant pleural mesothelioma (MPM). This is the first therapeutic approval in 15 years for MPM.
NovoTTF-100L is a tumor-treating fields (TTF) device, which uses electric fields to disrupt solid tumor cancer cell division. The device is approved for use in combination with pemetrexed plus a platinum-based chemotherapy—pemetrexed plus cisplatin being the only approved therapy for patients with unresectabled MPM. In an effort to promote therapeutic innovation for rare diseases, the Humanitarian Device Exemption—the approval path for the NovoTTF-100L—does not require evidence of efficacy. However, data from the STELLAR trial, a prospective, single-arm trial of NovoTTF-100L plus chemotherapy in 80 patients with unresectable MPM showed no serious systemic adverse events for the device, with mild-to-moderate skin irritation being the most common adverse event. Of the 53 patients with epithelioid MPM, median OS was 21.2 months; median OS was 12.1 months for the 21 patients with non-epithelioid MPM. At 12 months, 62% of all patients were alive, and the ORR was 40%. The median PFS was 7.6 months. Further studies are needed to determine the efficacy of this device. Until phase III data are available to document superiority compared with chemotherapy alone, it is unclear how readily this device will be embraced in the United States.
Expanded Indication for Pembrolizumab
April 11, 2019—The U.S. Food and Drug Administration (FDA) expanded the approval of pembrolizumab in the first-line setting to patients with PD-L1 expression of ≥ 1% (tumor proportion score [TPS]), as determined by an FDA-approved assay. This includes patients with unresectable stage III NSCLC who are not candidates for definitive chemoradiation as well as patients with metastatic NSCLC. This indication excludes EGFR-/ALK-positive NSCLC.
Pembrolizumab is already approved as a single agent for the first-line treatment of patients with metastatic NSCLC and PD-L1 expression ≥ 50% (TPS) and as combined with platinum-based doublet (carboplatin and pemetrexed) regardless of PD-L1 expression. Recent approval was based on KEYNOTE-042, a randomized, multicenter, open-label, active-controlled trial conducted in 1,274 patients with stage III or IV NSCLC. Chemotherapy-naive patients with PD-L1 expression of > 1% (TPS) received either 200 mg IV of pembrolizumab every 3 weeks or investigator’s choice of a carboplatin- containing regimen with either pemetrexed or paclitaxel. Patients were stratified by ECOG performance status, geographic region, histology, and PD-L1 expression (TPS > 50% or TPS 1%-49%).
Median OS was 16.7 vs 12.1 months for pembrolizumab vs chemotherapy, respectively, in those patients with PD-L1 expression > 1% (HR 0.81; 95% CI: 0.71, 0.93; p = 0.0036). For those patients with PD-L1 expression ≥ 20%, the median OS was 17.7 months and 13.0 months, respectively (HR 0.77; 95% CI: 0.64, 0.92; p = 0.004). The estimated median OS was 20 months vs 12.2 months, respectively, for patients with PD-L1 expression > 50% (HR 0.69; 95% CI: 0.56, 0.85; p = 0.0006).
However, in an exploratory analysis, in the cohort of patients with PD-L1 expression of 1%-49%, the median OS was 13.4 months for pembrolizumab vs 12.1 months for chemotherapy (HR 0.92; 95% CI: 0.77, 1.11). Hence, the positivity of this trial was driven by the results observed in patients with tumor PD-L1 expression levels of 50% or higher.
Editor’s Note: The approval of single-agent pembrolizumab in patients with NSCLC with tumor PD-L1 expression levels of 1% to 49% remains controversial. As delineated by the exploratory analysis presented by Gilberto Lopes at the 2018 American Society of Clinical Oncology Annual Meeting, pembrolizumab did not result in an obvious survival advantage in this cohort compared to conventional platinum-based chemotherapy in treatment-naive patients. For now, based on the survival results of KEYNOTE-189 and KEYNOTE-407, pembrolizumab in combination with histology-appropriate chemotherapy remains the standard of comparison in this population. The National Clinical Trials Network in the United States is about to launch a phase III trial directly comparing single-agent pembrolizumab to combination pemetrexed/carboplatin and pembrolizumab in patients with advanced nonsquamous NSCLC with any degree of PD-L1 expression. –Corey Langer, MD, Editor
First New Treatment for SCLC in 20 Years
March 19, 2019—The U.S. Food and Drug Administration approved atezolizumab for the first-line treatment of patients with previously untreated extensive-stage SCLC. The approval was based on data from the global, randomized, double-blind, placebo-controlled, phase III IMpower133 trial, which was presented at the IASLC 2018 World Conference on Lung Cancer. Data showed that the addition of concurrent and maintenance atezolizumab to first-line carboplatin and etoposide resulted in a significant overall survival benefit. Median OS was 12.3 months with atezolizumab versus 10.3 months in the control arm (hazard ratio [HR] for death 0.70 [0.54, 0.91]). In addition, the 1-year overall survival rate was 51.7% in the atezolizumab group and 38.2% in the placebo group. The objective response rates were 60% and 64%, respectively. For more on the clinical and research implications resulting from IMpower133, read the IASLC Lung Cancer News article by the study author, Dr. Stephen V. Liu.
European Commission Approval of Atezolizumab in Combination with Bevacizumab and Chemotherapy
March 8, 2019—The European Commission approved the combination of atezolizumab, bevacizumab, and chemotherapy for first-line treatment of patients with metastatic non-squamous NSCLC. The approval was based on the significant survival benefit seen in the phase III IMpower150 trial for the combination of atezolizumab, bevacizumab, paclitaxel, and carboplatin (ABPC) compared with BPC alone (median overall survival [OS] = 19.8 vs 14.9 months; hazard ratio [HR] = 0.76; 95% CI: 0.63–0.96; p = 0.006). The combination has already been approved by the U.S. Food and Drug Administration. For a more nuanced perspective on the FDA’s approval, read the jointly authored perspective by the IASLC Lung Cancer News Editorial Group, “Thoughts on IMpower 150: Latest FDA Approval for Atezolizumab Misses the Mark.”
European Medicines Agency Approves Lorlatinib
March 1, 2019—The EMA has endorsed lorlatinib for the treatment of patients with ALK-positive advanced NSCLC that has progressed during prior kinase inhibitor therapy. The Committee for Medicinal Products for Human Use recommended granting of a conditional marketing authorization. Lorlatinib is recommended as monotherapy for patients who disease has progressed after first-line treatment with alectinib or ceritinib, or with crizotinib plus at least one other ALK-based TKI.
Lorlatinib was approved by the U.S. Food and Drug Administration in November 2018.
FDA Grants Priority Review to Roche’s Personalized Medicine Entrectinib
February 19, 2019—The U.S. Food and Drug Administration (FDA) accepted a New Drug Application (NDA) and granted Priority Review for entrectinib for treatment of patients with ROS-1 mutations and metastatic NSCLC. The FDA is expected to make a final decision regarding approval by mid-August 2019. Entrectinib also has received the FDA’s Breakthrough Therapy Designation, Priority Medicines designation by the European Medicines Agency (EMA), and Sakigake designation by the Japanese health authorities for the treatment of NTRK fusion-positive locally advanced or metastatic solid tumors in adult or pediatric patients who have experienced disease progression following prior therapies or have no other treatment options.
E-cigs Help Smokers Quit, Health Risks Unknown
January 30, 2019—British researchers found that e-cigarettes were more effective for smoking cessation than nicotine-replacement therapy when combined with behavioral therapy. The randomized trial, the results of which were published in The New England Journal of Medicine on January 30, found an almost double sustained abstinence rate after 1 year among the smokers randomly assigned to the e-cigarette group: 18.0% vs. 9.9% for nicotine-replacement products (relative risk 1.83; 95% CI: 130-2.58, p < 0.001). The study had several limitations, however, and the rate of continued e-cigarette use was fairly high. E-cigarettes may pose health risks, the severity of which are unknown, so long-term use could be problematic.
Genomic Profile Test Approved in Japan
December 27, 2018—The Ministry of Health, Labour, and Welfare (MHLW) approved the FoundationOne CDx as a comprehensive genomic profiling test for all solid tumors and as a companion diagnostic for patients with advanced cancer. Similar to the FDA approval in the United States, MHLW reimbursement coverage is expected to include MHLW-approved targeted therapies and immunotherapies therapeutic selection based on FoundationOne CDx results.
New Approval for Atezolizumab
December 6, 2018—The U.S. Food and Drug Administration (FDA) approved atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin for management of metastatic nonsquamous NSCLC with no EGFR or ALK mutations in the first-line setting. Atezolizumab is also approved by the FDA for treatment of patients with metastatic NSCLC who experience disease progression during or following treatment with platinum-based chemotherapy, as well as for those patients who have EGFR or ALK mutations and have experienced disease progression during or after targeted therapy.
CheckMate-451 OS Endpoint Not Met
November 26, 2018—The phase III CheckMate-451 trial did not meet its primary endpoint of OS with combination nivolumab/ipilimumab vs. placebo as maintenance therapy for patients with extensive-stage SCLC with stable disease or response after first-line therapy with a platinum-based chemotherapy. Specific data are forthcoming, including data regarding the secondary endpoint of OS with single-agent nivolumab vs. placebo.
MYSTIC Fails to Meet Improved OS Endpoint
November 16, 2018—Phase III OS results were announced for MYSTIC, a randomized, open-label, multicenter, international trial of durvalumab monotherapy vs. durvalumab plus tremelimumab, an anti–CTLA-4 antibody, vs. platinum-based chemotherapy in treatment-naive patients with metastatic NSCLC. In the primary analysis of patients with PD-L1 expression on 25% or more of their cancer cells (as determined by the VENTANA PD-L1 Sp263 Assay), neither durvalumab alone nor its combination with tremelimumab significantly improved survival compared with chemotherapy. A hazard ratio of 0.76 (97.54% CI: 0.564-1.019; nominal p = 0.036) was observed for durvalumab alone; the combination’s HR was 0.85 (98.77% CI: 0.611-1.173; nominal p = 0.202).
Lorlatinib Approved for Second- or Third-Line Management of ALK-positive Metastatic NSCLC
November 2, 2018—The U.S. Food and Drug Administration granted accelerated approval to lorlatinib for patients with, ALK (+) metastatic or recurrent NSCLC with disease progression on crizotinib and at least one other ALK inhibitor or on alectinib or ceritinib as first ALK inhibitor therapy for metastatic or recurrent disease.
The accelerated approval was based on data from a nonrandomized, dose-ranging multicohort, multicenter study evaluating a subgroup of 215 patients with ALK-positive metastatic NSCLC who received prior treatment with one or more ALK kinase inhibitors (Study B7461001; NCT01970865). The major efficacy measures were overall response rate (ORR) and intracranial ORR, according to RECIST 1.1, as assessed by an independent central review committee.
The ORR was 48% (95% CI: 42-55), and the estimated median response duration was 12.5 months (95% CI: 8.4-23.7. Response rates were 4% for complete and 44% for partial responses. Of 89 patients with measurable CNS lesions (according to RECIST 1.1), the intracranial ORR was 60% (95% CI: 49-70), with 21% complete and 38% partial responses. The estimated median response duration was 19.5 months for those with measurable CNS lesions (95% CI: 12.4-not reached).
The most common adverse reactions (incidence ≥ 20%) were edema, peripheral neuropathy, cognitive effects, dyspnea, fatigue, weight gain, arthralgia, mood effects, and diarrhea.
Pembrolizumab/Carboplatin Plus Paclitaxel or Nab-Paclitaxel Approved for First-Line Treatment of Metastatic Squamous NSCLC
October 30, 2018—The U.S. Food and Drug Administration (FDA) approved pembrolizumab, an anti–PD-1 agent, in combination with carboplatin and either paclitaxel or nab-paclitaxel for the first-line treatment of patients with metastatic squamous NSCLC. The approval was based on the results of the KEYNOTE-407 trial, a pivotal phase III trial in which patients who received pembrolizumab in combination with carboplatin and either paclitaxel or nab-paclitaxel experienced significantly improved overall survival, regardless of PD-L1 expression status. Risk of death was reduced by 36% for patients receiving the pembrolizumab/ chemotherapy combination compared with chemotherapy alone (HR = 0.64; 95% CI: 0.49-0.85; p = 0.0017). This is the first approval for an anti–PD-1 regimen for this indication regardless of PD-L1 expression status.
Dacomitinib Approved as EGFR TKI
September 27, 2018—The U.S. Food and Drug Administration (FDA) approved dacomitinib for the first-line treatment of patients with metastatic NSCLC who have sensitizing EGFR mutations.
Approval was based on the ARCHER 1050 trial, which compared the safety and efficacy of dacomitinib to gefitinib in 452 patients with unresectable, metastatic NSCLC. Patients were required to have no prior therapy for metastatic disease or recurrent disease with a minimum of 12 months disease-free after completion of systemic non-EGFR TKI-containing therapy; an ECOG PS of 0 or 1; and EGFR exon 19 deletion or exon 21 L858R substitution mutations. Patients were randomly assigned to receive either 45 mg of dacomitinib orally once daily or 250 mg of gefitinib orally once daily until disease progression or unacceptable toxicity.
The trial showed that dacomitinib improved OS over gefitinib (34.1 vs. 26.8 months, p = 0.0438; HR 0.76, 95% CI [0.582-0.993]). For ARCHER 1050 details and for a more robust discussion of EGFR TKIs, read the article by Edgardo S. Santos Castillero, MD.
RET Inhibitor Wins Breakthrough Therapy Designation
September 5, 2018—The U.S. Food and Drug Administration n (FDA has granted LOXO-292, an investigational new agent, Breakthrough Therapy Designation for treatment of patients with RET-positive NSCLC who require systemic therapy and have experienced disease progression following treatment with a platinum-based chemotherapy and an anti–PD-1/PD-L1 therapy.
The designation was based on the phase I/II LIBRETTO-001 trial, an open-label, multi-center trial, which will be conducted in two parts: phase I (dose escalation) and phase II (dose expansion). The trial is currently enrolling patients. Patients are eligible if they have RET-fusion–positive NSCLC that has progressed on or if they have proven intolerant to available standard therapies.
New Standard of Care for First-line Management of EGFR mutation-positive NSCLC
August 21, 2018—The Japanese Ministry of Health, Labour, and Welfare approved osimertinib for first-line treatment of patients with inoperable or recurrent EGFR mutation-positive NSCLC. Approval was based on superior progression-free survival for osimertinib (18.9 months) compared with gefitinib or erlotinib (10.2 months), which was consistent across all subgroups, as found in the FLAURA trial. Osimertinib was also generally well tolerated, with grade 3 or higher adverse events occurring in 34% of patients (vs. 45% for gefitinib or erlotinib).
Japan is the 40th country to approve osimertinib for this indication.
Alectinib Approved for ALK-Positive NSCLC in China
August 20, 2018—The China National Drug Administration granted marketing authorization for alectinib as monotherapy for patients ALK-positive advanced NSCLC. This authorization follows on the heels of US Food and Drug Administration and European Medicines Agency approvals. Authorization was based on the phase III ALEX and ALESIA studies, as well as on two phase II studies assessing alectinib for treatment of patients with disease progression on or intolerance to crizotinib. Updated ALEX results were presented June 2018 at the American Society of Clinical Oncology meeting and showed that PFS more than tripled for patients who received alectinib vs. crizotinib (34.8 months vs. 10.9 months, respectively). Specifically regarding Asian patients with ALK-positive NSCLC, ALESIA found that alectinib reduced the risk of progression or death compared with crizotinib; this data will be submitted to the CDNA to complete a post-approval agreement. Results of ALESIA will be presented at an upcoming meeting.
Nivolumab Wins First Approval for Large Group of SCLC Patients
August 17, 2018—Nivolumab was granted approval by the US Food and Drug Administration for patients with metastatic SCLC whose cancer has progressed after platinum-based chemotherapy and at least one other line of therapy. This is the first approved therapy for this indication. Because this accelerated approval was based on data from the phase I/II CheckMate-032 for overall response rate and duration of response only, further confirmatory data may be required.
In CheckMate-032 109 patients received nivolumab after platinum-based chemotherapy and at least one other prior line of therapy. Of these, 13 responded (12%), with one complete response. In a separate analysis of 245 patients with SCLC and disease progression after chemotherapy, 45% had serious adverse reactions to nivolumab, the most common being fatigue, decreased appetite, musculoskeletal pain, dyspnea, nausea, diarrhea, constipation, and cough.
Lurbinectedin Receives Orphan Drug Designation
August 3, 2018—The U.S. Food and Drug Administration (FDA) has granted Orphan Drug designation to lurbinectedin for the treatment of SCLC. Lurbinectedin (PM1183) is an investigational drug that inhibits RNA polymerase II, which is essential for transcription-addicted tumors. It is being investigated in the salvage setting for patients whose disease has progressed after initial treatment.
Europe Closes Gap to Approval of Anti–PD-1, Chemo Combination
July 30, 2018—The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion regarding approval of pembrolizumab, an anti–PD-1 therapy, in combination with pemetrexed and platinum chemotherapy (cisplatin or carboplatin) in the first-line setting for patients with metastatic nonsquamous NSCLC who do not harbor EGFR or ALK mutations, regardless of PD-L1 expression.
If approved, this would mark the first approval in Europe for anti–PD-1 therapy in combination with chemotherapy. Approval decisions are being based on OS and PFS data from KEYNOTE-189.
FDA Grants Priority Review for sBLA for Pembrolizumab
July 2, 2018—The U.S. Food and Drug Administration (FDA) granted a priority review for a supplemental biologics license application (sBLA) for pembrolizumab. The application is based on based on results of the phase III KEYNOTE-189 trial and seeks approval for pembrolizumab in combination with pemetrexed and platinum chemotherapy (carboplatin or cisplatin) as a first-line treatment for patients with metastatic NSCLC, regardless of PD-L1 expression. The FDA has set a Prescription Drug User Fee Act date of September 23, 2018.
CNDA Approves Nivolumab, China’s First PD-1 Inhibitor
June 15, 2018—the China National Drug Administration (CNDA) approved nivolumab injection for the treatment of locally advanced or metastatic NSCLC after prior platinum-based chemotherapy for patients without EGFR or ALK tumor mutations. This is China’s first and only PD-1 inhibitor. Approval was based on data from the phase III CheckMate-078 trial, in which 90% of the patients enrolled were Chinese.
ALEX Study Leads to Health Canada’s Approval of Alectinib
June 13, 2018—Health Canada approved alectinib as a monotherapy for the first-line treatment of patients with ALK-positive locally advanced (not amenable to curative therapy) or metastatic NSCLC. The approval was based on results from the phase III ALEX study, which demonstrated a reduced risk of disease progression or death by more than half (53%) with alectinib versus crizotinib. ALEX data also showed that alectinib reduced the risk of metastasis to the brain or CNS by 84% compared with crizotinib.
Medicare Approves Liquid Biopsy for NSCLC
June 12, 2018—The Guardant360® assay, a liquid biopsy by Guardant Health, Inc., has been approved for all fee-for-service Medicare patients with metastatic NSCLC who meet certain criteria. Approval was based on a recent study by Odegaard et al. of 10,593 patients with advanced solid tumors that showed high feasibility (> 99.6%) and clinical sensitivity (85.9%), with high potential actionability (72% with treatment or trial recommendations). This was especially true for patients with NSCLC; 34.5% of patient samples contained a directly targetable standard-of-care biomarker.
European Commission Grants Marketing Authorization for Osimertinib
June 8, 2018—The European Commission granted marketing authorization in early June 2018 for osimertinib as monotherapy for the first-line treatment of patients with locally-advanced or metastatic NSCLC with activating EGFR mutations. The approval is based on results from the phase III FLAURA trial.
The approval follows the positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency.
Crizotinib Receives Breakthrough Therapy Designation
May 29, 2018—A breakthrough therapy designation was granted to crizotinib, a kinase inhibitor, by the FDA for the treatment of patients with metastatic NSCLC with MET exon 14 alterations who experience disease progression after receiving platinum-based chemotherapy. Crizotinib is currently approved by the FDA for the treatment of patients with ALK- or ROS1-positive metastatic NSCLC.
Medtronic plc and Royal Philips Jointly Developing LungGPS™ Platform
January 30, 2018—Medtronic plc and Royal Philips are jointly developing and marketing the LungGPS™ Patient Management Platform. The LungGPS platform is designed to make it easier for clinicians to identify and manage patients with incidental pulmonary nodules within disparate hospital information systems.
The platform uses Natural Language Processing, a type of artificial intelligence technology that quickly searches and analyzes data contained within various medical reports and highlights relevant information for further review and follow up; it also uses Philips’ Cancer Screening Solution, a software system that automates routine administrative tasks and standardizes clinical workflows for optimized efficiency and patient care.











