Natural disasters, such as Hurricane Maria, may have contributed to more acute shortages than previously experienced.
By Jorge Garcia, PharmD, MS, MHA, MBA, and Dina B. Dumercy, PharmD, BCOP, BCPS
Posted: June 2018
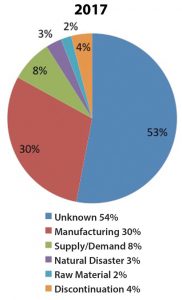
Drug shortages in oncology date back to the late 1980s, when there was a scarcity of paclitaxel. Since then, with an increase in the number of therapeutic options and the evolution of the market dynamics, and further compounded by the need for intravenous (IV) fluids to deliver chemotherapy/ immunotherapy, healthcare professionals face continuous drug-shortage challenges regarding the treatment of their patients with cancer. Mechanisms often responsible for shortages include: ingredient shortage, drug recalls, and low revenue margins (all of which discourage production); lack of ability to stockpile; tougher manufacturing standards (which puts some manufacturers out of compliance); industry consolidation; and natural disasters (Fig. 1).
Figure 1. Reasons for Shortages as Determined by UUDIS During Investigation
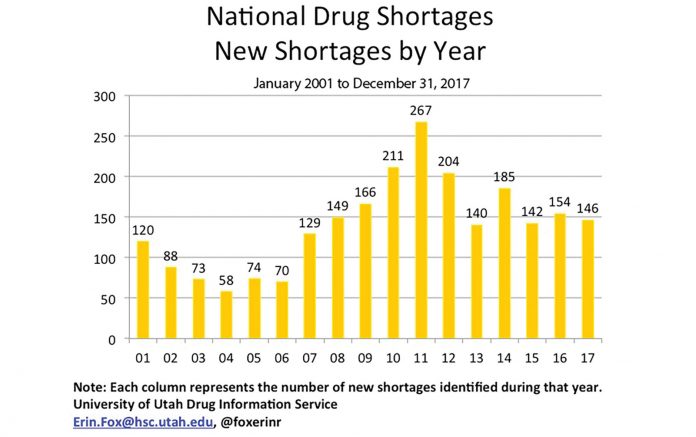
As a result, oncology healthcare professionals face significant moral and ethical dilemmas when having to make decisions regarding treatment interruption, transitions to less-effective treatments, or rationing of life-saving treatments.1 Although the current number of drug shortages is not the highest seen in recent years (Fig. 2), the industry has witnessed significant effects with regard to specific types of products (e.g., IV fluids) facing severe shortages. Strategies to successfully navigate the drug shortages must be multidimensional in approach, including careful management of supply and inventory, enhanced communication with stakeholders and suppliers, and operation within a safe and ethical framework. Drug shortages have created the need to evaluate alternative treatment options and administration strategies, as well as renewed attention on how to mitigate threats to clinical trials. Furthermore, with more pronounced drug shortages, there are major concerns regarding the effects on clinical outcomes associated with the treatment changes.
A Complex Universe of Factors
The U.S. Food and Drug Administration (FDA), the American Society for Health-System Pharmacists (ASHP), and numerous other organizations have provided recommendations on the management of supply and inventory. Although general, these recommendations apply across multiple specialties, including oncology. The transition to the use of IV push, when appropriate, can be made safer by the institution of specific administration instructions, provision of ready-to administer dosage forms to nurses, and flexibility within the electronic record to facilitate ease of ordering and conversion of fluids. Researchers and pharmacists have the opportunity to critically evaluate the design of clinical trials to determine the feasibility of drug substitutions and to minimize the waste associated with products on shortage. For example, this can be accomplished by asking study sponsors to provide supplies needed to prepare investigational medications, including fluids, or by requesting assistance with accessing critically short medications by alternative means. In the hospital or in large oncology practice settings, moving stock supplies to a central location for closer inventory control and to support provision of medication in the final solution for administration may minimize waste. The use of technology such as syringe pumps can help support appropriate administration duration for agents that cannot be given rapidly, minimizing the use of the IV fluid bags.
With the rapidly changing availability of medication, the new paradigm is to provide constant updates and to facilitate close communication between the prescribers, pharmacists, nurses, patients, and suppliers. With agents that are available with multiple dosage forms, when one formulation is on shortage, alternative options often become rapidly unavailable. In January 2018, this was experienced acutely with etoposide when, within minutes, wholesalers ran out of products that were identified as alternatives. This had immediate consequences. For example, in patients with SCLC, this meant an acute need to reassess alternatives to our standard therapy, cisplatin/etoposide. Corey Langer, Editor of the IASLC Lung Cancer News indicates that although his institution seemed to be shielded initially from this problem, that is no longer the case, at least as of February 2018; he and his colleagues, like many others, are now contending with the same issue.
One of the more challenging aspects of dealing with medications in limited supply is how to determine which patients warrant higher priority to receive the medications in question. In 2012, the Ontario Ministry of Health and Long-Term Care published the Ethical Framework for Resource Allocation During the Drug Supply Shortage.2
Dr. Jorge Garcia
 This framework promotes the use of ethical principles to make recommendations, such as ensuring standard of care and best practice whenever possible, using alternative treatments with similar benefits, exercising solidarity in sharing resources across health sectors, and distributing the drug in short supply to those who are in greatest need and who are most likely to benefit. It also recommends that the allocation of strategies be based on the clinical situation, be nondiscriminatory (e.g., not based on social status), and be geared to minimize any possible waste.
This framework promotes the use of ethical principles to make recommendations, such as ensuring standard of care and best practice whenever possible, using alternative treatments with similar benefits, exercising solidarity in sharing resources across health sectors, and distributing the drug in short supply to those who are in greatest need and who are most likely to benefit. It also recommends that the allocation of strategies be based on the clinical situation, be nondiscriminatory (e.g., not based on social status), and be geared to minimize any possible waste.
Dr. Dina B. Dumercy

Medication shortages affect institutions differently based on geographic location, existing contracts, access to secondary and tertiary wholesalers, allocations, and patient population, among other factors. Designing mitigation plans and executing such plans from a clinical and operational perspective, along with monitoring intended and unintended outcomes, incur significant costs associated with clinicians’ time and other financial resources. Although we hope the end of drug shortages is on the horizon, current mitigation efforts should focus on strategies to minimize waste, support proactive communication, and promote best practices. ✦
Editor’s Note
 In SCLC, simply substituting oral etoposide for intravenous is not necessarily desirable. The available literature suggests increased toxicity with potentially less efficacy. Fortunately, there are number of studies, including two phase III trials, one led by Nasser Hanna1 and another led by Primo Lara,2 that have shown de facto therapeutic equivalence between cisplatin/etoposide and cisplatin/irinotecan with similar response, progression-free, and overall survival rates for patients with extensive stage SCLC. In other situations, with other agents, such substitutions may not be so easy.
In SCLC, simply substituting oral etoposide for intravenous is not necessarily desirable. The available literature suggests increased toxicity with potentially less efficacy. Fortunately, there are number of studies, including two phase III trials, one led by Nasser Hanna1 and another led by Primo Lara,2 that have shown de facto therapeutic equivalence between cisplatin/etoposide and cisplatin/irinotecan with similar response, progression-free, and overall survival rates for patients with extensive stage SCLC. In other situations, with other agents, such substitutions may not be so easy.
–Corey Langer, MD, Editor
1. Hanna N1, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24(13):2038-2043.
2.Lara PN Jr1, Natale R, Crowley J, et al. Phase III trial of irinotecan/ cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27(15):2530-2535.
 Shortages in both chemotherapy drugs and reconstitution solutions (e.g., normal saline, dextrose 5% water) are posing tremendous challenges to clinicians: ethical issues and best clinical practice are compromised. Oncologic patients may not only be precluded from receiving standard-of-care therapy but also from the opportunity to participate in clinical trials. Recently, we have been forced to use oral etoposide, as well as irinotecan, in the first line for SCLC. As a result, one of my patients developed cisplatin/irinotecan-induced syndrome of inappropriate antidiuretic hormone secretion (only one report in literature), and now that patient faces a third change in his first-line therapy. The lack of D5W solution has kept us from enrolling patients in an SCLC clinical trial. Initiatives must be undertaken at the federal level, through legislation along with pressure on manufacturers, to mitigate this huge healthcare problem as soon as possible. Importing products from overseas and extending expiration dates are among the safe ideas being discussed.
Shortages in both chemotherapy drugs and reconstitution solutions (e.g., normal saline, dextrose 5% water) are posing tremendous challenges to clinicians: ethical issues and best clinical practice are compromised. Oncologic patients may not only be precluded from receiving standard-of-care therapy but also from the opportunity to participate in clinical trials. Recently, we have been forced to use oral etoposide, as well as irinotecan, in the first line for SCLC. As a result, one of my patients developed cisplatin/irinotecan-induced syndrome of inappropriate antidiuretic hormone secretion (only one report in literature), and now that patient faces a third change in his first-line therapy. The lack of D5W solution has kept us from enrolling patients in an SCLC clinical trial. Initiatives must be undertaken at the federal level, through legislation along with pressure on manufacturers, to mitigate this huge healthcare problem as soon as possible. Importing products from overseas and extending expiration dates are among the safe ideas being discussed.
–Edgardo Santos, MD, IASLC Publications Committee Chair
Resources:
• Shortage Resources. American Society of Health- System Pharmacists website. ashp.org/Drug-Shortages/Shortage-Resources. Accessed February 3, 2018.
• FDA Drug Shortages. U.S. Food and Drug Administration website. www.accessdata.fda.gov/scripts/drugshortages. Accessed February 3, 2018.
References:
1. Jagsi R, Spence R, Rathmell K, et al. Ethical Considerations for the Clinical Oncologist in an Era of Oncology Drug Shortages. Oncologist. 2014;19(2):186-192.
2. Ethical Framework for Resource Allocation During the Drug Supply Shortage. Ontario Ministry of Health and Long-Term Care website. health.gov.on.ca/en/pro/programs/drugs/supply/docs/ethical_framework.pdf Accessed February 3, 2018.