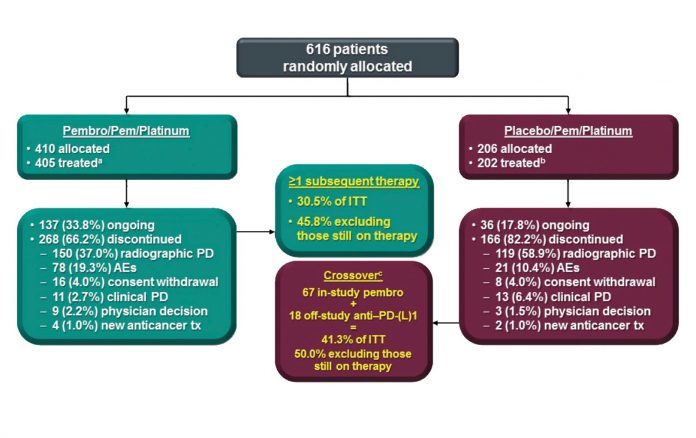
a AEs, 1 clinical PD, 1 death, and 1 protocol violation. Reprinted with permission from Merck.
By Corey Langer, MD, IASLC Lung Cancer News Editor
Posted: June 2018
The therapeutic landscape irrevocably altered on April 17, 2018, when three pivotal trials—KEYNOTE 189, CheckMate 227 and Impower 150—were presented during a plenary session at the American Association for Cancer Research (AACR) Annual Meeting in Chicago. As of that point, immunotherapy (IO) had not yet established primacy in the management of treatment-naive advanced NSCLC, but these trials have cemented its role.
KEYNOTE 189: Pembrolizumab Makes Waves
Leena Gandhi, MD, PhD, on behalf of the investigators of KEYNOTE 189, presented the results of a randomized phase III trial isolating the role of pembrolizumab in combination with pemetrexed and a platinum-based drug in patients with advanced nonsquamous NSCLC in the absence of EGFR or ALK alterations.1 Patients were randomly assigned 2:1 to either the pembrolizumab triplet or to chemotherapy/placebo for four cycles, after which patients on the investigational arm received maintenance therapy with pemetrexed and pembrolizumab, whereas the control group received pemetrexed/placebo (Fig. 1). At the time of disease progression, patients in the control group were offered crossover therapy to pembrolizumab. A total of 616 patients were enrolled. Those who received pembrolizumab attained a statistically significant and clinically meaningful improvement in overall survival (OS), with an unprecedented hazard ratio (HR) of 0.49 (Fig. 2, Fig 3). Median OS was not reached in the pembrolizumab arm compared to 11.3 months for the pemetrexed/placebo group; the respective 1-year survival rates were 69% and 49% (HR 0.49, 95% CI [0.38, 0.64]; p < 0.0001) with median progression-free survival (PFS) of 8.8 and 4.9 months, respectively (HR 0.52). The response rate in the investigational arm was 48% vs. 19% in the control arm (p < 0.00001). This benefit was observed across the board regardless of PD-L1 expression status, although the magnitude of benefit was more pronounced in those with higher levels of PD-L1 expression. For those with PD-L1 expression tumor proportion scores (TPS) of 50% or higher, the rate of response in the investigational arm was 62% vs. 23% in the control arm (HR 0.42, p < 0.0001). For TPS 1% to 49% and TPS < 1%, response rates were 48% vs. 21% (HR 0.55, p < 0.0001) and 32% vs. 14% (HR 0.59, p < 0.0001), respectively. Toxicities, particularly kidney injury, were heightened in the experimental arm but were manageable. Treatment discontinuation for adverse events (AEs) occurred in 13.8% of patients in the investigational arm compared to 7.9% in the control arm. Immune-related AEs occurred in 22.7% of patients in the investigational arm vs. 11.9% in the control arm.
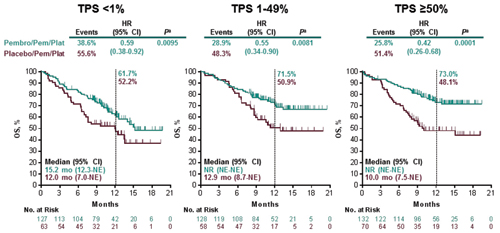
a Nominal and one-sided. Data cutoff date: Nov 8, 2017. Gandhi KN189 AACR 2018. Reprinted with permission from Merck.
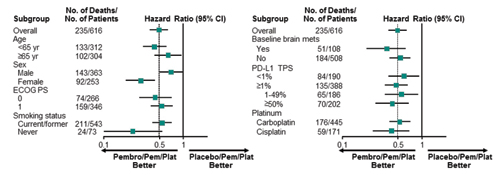
Data cutoff date: Nov 8, 2017. Reprinted with permission from Merck.
This study has upended standard practice in the United States and promises to have a ripple effect globally. There are many unanswered questions, however. Pembrolizumab is approved as a single agent in advanced NSCLC for use with PD-L1 expression levels of 50% or higher based on the results of KEYNOTE 024, which showed superiority to chemotherapy in this setting.2 KEYNOTE 189 did not compare the efficacy of combination pembrolizumab and chemotherapy to pembrolizumab alone, however. This point remains controversial, and its urgency is amplified by a recent press release on KEYNOTE 042, which reportedly demonstrated improved OS for single-agent pembrolizumab vs. chemotherapy in patients with any degree of PD-L1 expression.3 Fortunately, the upcoming INSIGNIA trial—as outlined by Roy S. Herbst, MD, PhD, the discussant of KEYNOTE 189 at AACR—will address the issue of sequencing, directly comparing single-agent pembrolizumab in PD-L1–positive nonsquamous NSCLC to the three-drug combination; patients receiving pembrolizumab at the time of disease progression will go onto chemotherapy alone (pem/carbo) or on to a combination of pembrolizumab with chemotherapy.4 In addition, KEYNOTE 189 did not address the role of IO/chemotherapy combinations in those with EGFR mutations or ALK translocations or in those patients eligible for angiogenesis inhibition.
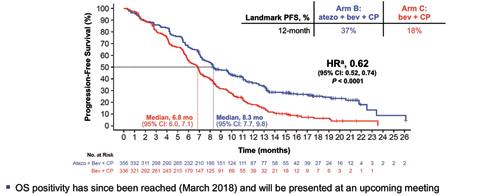
Abbreviations: atezo, atezolizumab; bev, bevacizumab: CP, carboplatin + paclitaxel. a Stratified HR. Data cutoff : September 15, 2017. Reck M, et al. ESMO IO 2017 [LBA_PR1]. Kowanetz M, Socinski M, et al. AACR 2018 IMpower150: Efficacy Across Subgroups. Reprinted with permission from Socinski M.
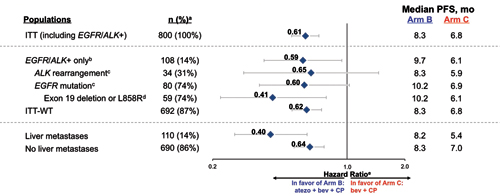
Abbreviations: atezo, atezolizumab; bev, bevacizumab; CP, carboplatin + paclitaxel. a Prevalence % for ITT, EGFR/ALK+ only, ITT-WT, liver metastases, and no liver metastases out of ITT (n=800); prevalence % for ALK rearrangement and EFGR mutation out of EGFR/ALK+ only (n=108); prevalence % for exon 19 deletion of L858R out of EFGR mutation (n=80). b Patients with a sensitizing EFGR mutation or ALK translocation must have disease progression or intolerance of treatment with one or more approved targeted therapies. c 6 patients had both EFGR mutation and ALK rearrangement. d Other EFGR mutations include L861Q, G719X, S7681, exon 20 insertion, T790M, and other. e Stratified HRs for ITT and ITT-WT populations; unstratified HRs for all other subgroups. Data cutoff : September 15, 2017 Kowanetz M, Socinski M, et al. AACR 2018 IMpower150: Efficacy Across Subgroups. Reprinted with permission from Socinski M.
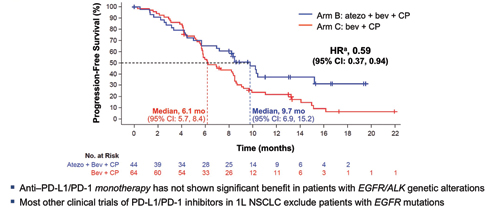
Abbreviations: atezo, atezolizumab; bev, bevacizumab: CP, carboplatin + paclitaxel. a Unstratified HRs. Data cutoff : September 15, 2017 Kowanetz M, Socinski M, et al. AACR 2018 IMpower150: Efficacy Across Subgroups. Reprinted with permission from Socinski M.
Impower150: Relevance for Oncogenic Drivers
In this regard, IMpower150, which was presented by Mark Socinski, MD, fills a void.5 Up until the emergence of IO in advanced NSCLC, the combination of paclitaxel and carboplatin with bevacizumab was considered by many a “state-of-the-art” regimen in advanced non-squamous NSCLC. The study E4599 demonstrated therapeutic superiority with improved response rates, PFS, and OS for this three-drug regimen compared to paclitaxel/carboplatin alone.6 In IMpower 150, eligible patients were randomly assigned to the E4599 regimen of paclitaxel/carboplatin and bevacizumab or the same regimen in combination with atezolizumab. A third arm substituted atezolizumab for bevacizumab.
More than 1,200 patients were enrolled on this trial. In the intent-to-treat wildtype population, 356 patients randomly assigned to the four-drug atezolizumab arm realized a median PFS of 8.3 months and 1-year PFS of 37% compared to 6.8 months and 18%, respectively, for the 336 patients on the control arm (Figs. 4-6; HR 0.62, 95% CI [0.52, 0.74]; p < 0.0001).
As we observed in KEYNOTE 189, those with higher levels of PD-L1 expression had relatively greater benefit; the HR in the 20% of patients with very high levels of PD-L1 expression was 0.39, with median PFS of 12.6 vs 6.8 months for the atezolizumab combination and the control group, respectively. In addition, the PFS benefit was similar for those with EGFR/ALK alterations, with an HR of 0.59 and median PFS of 9.7 and 6.1 months, respectively. In those with “actionable” EGFR mutations, the PFS benefit was even more pronounced with an HR of 0.41 and a PFS of 10.2 and 6.1 months, respectively.
A recent press release confirmed an OS advantage for the four-drug regimen.7 At a related presentation at the ESMO Immuno Oncology Congress, the median OS was 19.2 months for the atezolizumab arm compared to 14.4 months for the control arm (HR 0.775, 95% CI [0.619, 0.970]; p = 0.0262). Given the results of KEYNOTE 189, it is unclear whether there will be much uptake for the four-drug regimen due to its complexity and the inherent toxicities of taxanes, including hair loss and neuropathy. However, this study is unique in addressing patients with oncogenic drivers and has clinical relevance for those whose disease is TKI refractory.
Dr. Socinski told IASLC Lung Cancer News that this trial “tests the theory that VEGF inhibition may augment the effectiveness of anti–PD-L1 therapy. The trial provides validation for this strategy, creating a new option for bevacizumab-eligible patients, particularly in certain subsets such as EGFR mutated and ALK-translocated patients.”
CheckMate 227: Using TMB to Determine Therapy
Finally, there are many investigators who firmly believe that IO combinations will ultimately displace chemotherapy in the management of advanced NSCLC. CheckMate 227, presented by Matthew D. Hellmann, MD, provides evidence that such an approach may have substantial merit in those with high tumor mutation burden (TMB) defined as > 10 mutations (mut)/Mb.8 In this study, patients with high TMB who received combination nivolumab and ipilumumab (nivo/ipi) had significantly improved PFS compared to patients who received standard platinumbased chemotherapy (HR 0.58, 95% CI [0.41, 0.81]; p < 0.001). This prespecified cohort was part of a much larger study in which individuals with chemotherapynaive stage IV or recurrent NSCLC and no known sensitizing EGFR or ALK alterations were enrolled in two groups based on PD-L1 expression. Patients with ≥ 1% PD-L1 expression were randomly assigned 1:1:1 to nivo/ipi, nivo alone, or to platinum-based chemotherapy; patients with < 1% expression were randomly assigned 1:1:1 to nivo/ipi, nivo combined with platinum-based chemotherapy, or to chemotherapy alone. Patients were treated until disease progression or unacceptable toxicity, with a maximum treatment period of up to 2 years. TMB was determined from tumor tissue using the validated FoundationOne CDx assay. The co-primary endpoint included PFS by blinded independent central review for nivo/ipi vs. chemotherapy for patients with TMB > 10 mut/Mb. At a minimum follow-up of 11.5 months, PFS was significantly longer for the nivo/ipi group vs. the control group in patients with high TMB (median PFS of 7.2 months vs. 5.4 months and 1-year PFS of 43% vs. 13%, respectively (Figs. 7 and 8); HR 0.58, 95% CI [0.41, 0.81]; p = 0.0002). This benefit was observed regardless of tumor histology, PD-L1 expression level, age, or gender. There was similar degree of benefit in both PD-L1 negative and PD-L1 positive patients (HR 0.48 and HR 0.62, respectively). Conversely, in patients with TMB < 10 Mut/MB, the HR for PFS was 1.07 (95% CI [0.84, 1.35]). Objective overall response rate was 45% for the nivo/ipi group vs. 27% for the control group for patients with TMB > 10 mut/Mb. Preliminary median OS favored the nivo/ipi arm at 23 vs. 16.4 months, respectively, with 1-year OS of 67% vs. 58%; this difference was not yet significant (HR 0.79, 95% CI [0.56, 1.10]).
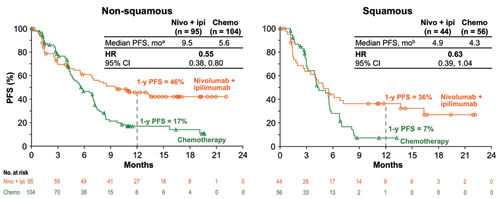
a 95% CI: nivo + ipi (5.6 mo, NR), chemo 4.5, 7.0 mo); b 95% CI: nivo + ipi (2.7, 13.7 mo), chemo (3.2, 5.6 mo) CheckMate 227: Nivo + Ipi in 1L NSCLC With High TMB (≥10 mut/Mb). Reprinted with permission from Hellman MD.
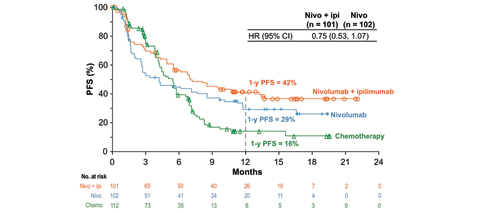
CheckMate 227: Nivo + Ipi in 1L NSCLC With High TMB (≥10 mut/Mb). Reprinted with permission from Hellman MD.
Others have voiced reservations regarding TMB. To paraphrase the comments of David L. Rimm, MD, PhD, the discussant of the study that has established 10 Mut/Mb as the threshold for TMB analysis (CheckMate 5689): TMB is provocative but not yet ready for prime time. Dr. Rimm indicated that, although there is a clear-cut correlation with PFS, we have not yet seen significant OS data. The test itself must be standardized. It costs 5 to 10 times more than immunohistochemistry (IHC) testing and requires a lot more tissue. In addition, as long as it depends on next-generation sequencing, results are generally delayed. All of these concerns are valid, but cost must be put into proper context. Although it is more expensive than IHC, next-generation sequencing, which helps determine TMB results, yields far more information than IHC, including crucial information on potential oncogenic drivers. In addition, its cost pales in comparison to the cost of a single cycle of single-agent immunotherapeutics or the empiric combination of pembrolizumab, pemetrexed, and carboplatin. ✦
References:
1. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. KEYNOTE-189: Randomized, Double-Blind Phase 3 Study of Pembrolizumab or Placebo Plus Pemetrexed and Platinum as First-Line Therapy for Metastatic NSCLC. Paper presented at: American Association for Cancer Research Annual Meeting; April 14-18, 2018; Chicago, IL.
2. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833.
3. KEYTRUDA® (pembrolizumab) Monotherapy Met Primary Endpoint in Phase 3 KEYNOTE-042 Study, Significantly Improving OS as First-Line Therapy in Locally Advanced or Metastatic NSCLC Patients Expressing PD-L1 in at Least 1 Percent of Tumor Cells. Published April 9, 2018. Accessed April 27, 2018.
4. Herbst R. In discussion of: Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. KEYNOTE-189: Randomized, Double-Blind Phase 3 Study of Pembrolizumab or Placebo Plus Pemetrexed and Platinum as First-Line Therapy for Metastatic NSCLC. Paper presented at: American Association for Cancer Research Annual Meeting; April 14-18, 2018; Chicago, IL.
5. Kowanetz M, Socinski MA, Zou W, et al. IMpower150: IMpower150: Efficacy of Atezolizumab Plus Bevacizumab and Chemotherapy in 1L Metastatic Nonsquamous NSCLC Across Key Subgroups. Paper presented at: American Association for Cancer Research Annual Meeting; April 14-18, 2018; Chicago, IL.
6. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542-2550.
7. Phase III IMpower150 Study Showed Genentech’s TECENTRIQ (Atezolizumab) and Avastin (Bevacizumab) Plus Carboplatin and Paclitaxel Helped People With Advanced Lung Cancer Live Longer Compared to Avastin Plus Carboplatin and Paclitaxel. Published March 25, 2018. Accessed April 27, 2018.
8. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab + Ipilimumab vs Platinum-Doubled Chemotherapy as First-line Treatment for Advanced Non-Small Cell Lung Cancer: Initial Results from CheckMate 227. Paper presented at: American Association for Cancer Research Annual Meeting; April 14-18, 2018; Chicago, IL.
9. Rimm DL. In discussion of: Tumor Mutational Burden (TMB) as a Biomarker for Clinical Benefit from Dual Immune Checkpoint Blockade With Nivolumab + Ipilimumab in First-Line Non-Small Cell Lung Cancer: Identification of TMB Cutoff From CheckMate 568. Paper presented at: American Association for Cancer Research Annual Meeting; April 14-18, 2018; Chicago, IL.
Breaking News Brief
The FDA has accepted a supplemental Biologics License Application (sBLA) and granted Priority Review for atezolizumab, in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of people with metastatic nonsquamous NSCLC. The FDA is expected to make a final decision regarding approval in early September 2018. ✦ For more news about atezolizumab, see Breaking News Briefs.










