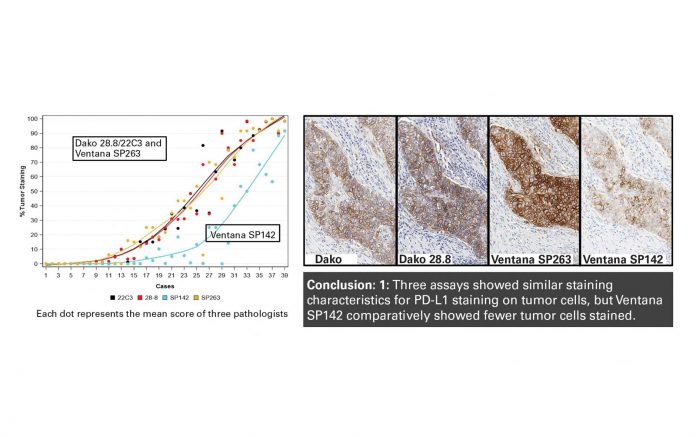
Reproduced with permission. Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phases 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thor Oncol. 2017;12(2) 208-222.
By Fred R. Hirsch, MD, PhD
Posted: April 2018
Immunotherapy is a very encouraging development in the treatment of patients with lung cancer. At this phase of its refinement, we struggle with the challenge of how to select the right patients for the right treatment. Patient selection is paramount because only 20% to 30% of patients with advanced NSCLC will benefit from immunotherapy.
PD-L1 protein expression has been the biomarker of choice for clinical trials. Based on trial results, PD-L1 immunohistochemistry (IHC) assays have been approved either as a companion diagnostic (e.g., pembrolizumab) or as a complementary diagnostic (e.g., nivolumab and atezolizumab). In some cases, biomarkers for PD-L1 are still under clinical exploration with other agents in clinical trials (e.g., durvalumab and avelumab). A clinical challenge is that each pharmaceutical company testing these agents in clinical trials is pursuing their own unique PD-L1 assay, each of which differs regarding antibody clones, staining platforms, and definitions of cutoff levels for positive versus negative status or for high PD-L1 expression levels versus low PD-L1 expression levels.
In an effort to provide consistency in this area, members of the IASLC pathology panel and other IASLC members proposed a comparative study of the performance of the various PD-L1 IHC assays. The study was encouraged by the U.S. Food and Drug Administration as well as by the American Association for Cancer Research and the American Society of Clinical Oncology. A steering committee consisting of representatives from multiple pharmaceutical companies (AstraZeneca, Genentech/ Roche, Bristol-Myers Squibb, Merck Pharmaceuticals, and, later, Pfizer and Merck MSD), and the two diagnostic companies (Ventana and Dako) was established, and the study was performed by members of the IASLC Pathology Committee and coordinated by IASLC.

Study Findings to Date
Phase I of the Blueprint Project was a feasibility study evaluating 39 tumors stained with four distinct antibodies: Dako 28-8 (for use with nivolumab), Dako 22C3 (for use with pembrolizumab), Ventana SP263 (for use with durvalumab), and Ventana SP142 (for use with atezolizumab). All staining was performed according to the standard procedure delineated in the clinical trials. The results from phase I were published in the Journal of Thoracic Oncology.1 The results showed that three assays (Dako 28-8/22C3 and Ventana SP263) yielded very similar PD-L1 expression levels on tumor cells, whereas Ventana SP142 stained fewer tumor cells (Figure 1). Regarding PD-L1 expression on immune cells, the variation among the different assays was much greater.
Phase II of the Blueprint Project also included Dako 73-10 (for use with avelumab). The goal of this phase was validation of the phase I observations, specifically regarding interobserver variability. Phase II included a larger panel of pathologists (25 from 15 countries) and examined a larger number of “real life” tumors (81 NSCLC tumors). The results from phase II were presented by Ming-Sound Tsao, MD, at the 2017 World Conference on Lung Cancer in Yokohama, Japan.2 Results verified that the three assays (Dako 28-8/22C3 and Ventana SP263) showed very similar levels of PD-L1 expression on tumor cells, whereas Ventana SP142—similar to what was seen in the phase I analysis— stained fewer tumor cells. The newer assay Dako 73-10 stained a slightly higher percentage of tumor cells. Furthermore, phase II demonstrated, in general, reasonably good interobserver concordance among the 25 pathologists. Comparison of PD-L1 expression based on tumor versus cytology specimens also showed good correlation and there was good correlation between PD-L1 assessment using glass slides (e.g., microscope) or digital scans.
Thus far, the PD-L1 Blueprint Project has underscored that interchangeability of the three assays (Dako 28-8/22C3 and Ventana SP263) is a possibility. However, the clinical cutoff s chosen for positive status/high expression levels versus negative status/ low expression levels might be the determining factor in clinical associations, rather than the actual choice of assays.
Phase IIB of the PD-L1 Blueprint Project is ongoing and will compare PD-L1 expression based on large tumor specimens versus smaller biopsies and cytologies from the same tumors.
Finding Scientific Value through Partnership
We hope the PD-L1 Blueprint Project will provide scientific evidence that interchangeability among at least some of the PD-L1 assays can be accomplished safely; it appears that only cutoff values for the definitions of low versus high PD-L1 expression (or positive versus negative status) may vary among the different PD-L1/PD-1 antibodies. If that is the case, PD-L1 assessment will be more easily and consistently determined, and we will gain a better understanding of its clinical importance. Furthermore, the Blueprint Project will determine the suitability of smaller biopsies and cytology specimens for PD-L1 expression evaluation, compared with larger specimens. Th s determination is critically important because the majority of patients with advanced lung cancer will have small biopsies or cytology samples as the primary diagnostic material.
It is the IASLC Blueprint team’s goal to apply all five of the PD-L1 assays to a well defined clinical cohort that is treated with an immunotherapy agent so that response rates and outcomes can be compared across assays in the same population.
As a capstone, The IASLC PD-L1 Blueprint Project has demonstrated the feasibility and value of having several industry partners (who, in the clinical world, would be considered competitors) working together toward common scientific goals. Although certain challenges had to be overcome, the Blueprint Project has gathered these industry partners around the same table, each agreeing to solve important clinical issues. It has been my pleasure as CEO of the IASLC to be the mediator of this initiative and to coordinate these studies. ✦
About the Author: Dr. Hirsch is CEO of the International Association for the Study of Lung Cancer and Professor of Medicine and Pathology at University of Colorado Cancer Center.
References:
1. Hirsch FR, McElhinny A, Stanforth D, et al. J Thorac Oncol. 2017 Feb;12(2):208-222.
2. Tsao MT, Kerr K, Yatabe Y, et al. PL 03.03 Blueprint 2: PD-L1 Immunohistochemistry Comparability Study in Real-Life, Clinical Samples. J Thor Oncol. 2017;12(Suppl 2):S1606.










