By Hossein Borghaei, MS, DO
Posted: February 2018
The role of checkpoint inhibitors in the management of recurrent or metastatic NSCLC is now well established. For patients who are treatment naive who do not have a driver mutation and who have a high PD-L1 expression (≥50% tumor proportion score), frontline pembrolizumab is the accepted standard of care based on the results of the KEYNOTE-024 study.1 Based on the results of KEYNOTE-021 cohort G, a randomized phase II trial, the U.S. Food and Drug Administration has approved the triple combination of carboplatin, pemetrexed, and pembrolizumab in all patients with non–squamous cell NSCLC, regardless of PD-L1 expression levels.2 The results of this trial indicate that the combination of a platinum-doublet chemotherapy and a checkpoint inhibitor is not only feasible from a safety standpoint, but it can also lead to better response rates and possibly better overall survival (OS) duration. However, many in the thoracic oncology community remain skeptical of the role of such combinations for first-line treatment of patients with advanced disease. In the absence of definitive phase III data, platinum-doublet chemotherapy remains a viable option for patients with less than 50% PD-L1 expression and no driver mutations.
Promising Results for OS, PFS
IMpower 150 is a randomized phase III trial designed to assess whether the addition of atezolizumab, a PD-L1 antibody, to a backbone of carboplatin, paclitaxel, and bevacizumab (C, P + B) could provide better clinical efficacy (Fig. 1). As reported by Reck et al., this combination resulted in an improvement in progression-free survival (PFS) in all patients, even those with driver mutations (Fig. 2).3 This is notable because prior immunotherapy studies that allowed patients with driver mutations (or that reported the demographic surrogate of never-smoker status) demonstrated either comparability or inferiority for this subgroup.
The survival data are not yet mature, but preliminary results indicate an improvement in OS with the four-drug combination (median OS of 19.2 vs. 14.4 months, HR: 0.77, p = 0.026). The reported results included the analysis of two of the three arms but did not include the full analysis of the third arm that featured C + P plus atezolizumab without bevacizumab.
At this time, there is no
convincing evidence that
combinations are consistently
improving OS in patients with
high PD-L1 expression.
PFS analysis of multiple subgroups indicates that this quadruple combination was more effective than the triplet combination. In the driver mutation–negative group, the median PFS improved from 6.8 to 8.3 months, and response rates improved to 64% from 48%. PFS benefit was seen across all PD-L1 levels, with PD-L1–high tumors (TC3 or IC3) showing the greatest improvement compared with TC/IC 0, 1, and 2 groups. However, even the TC/IC 0 group, which comprised 49% of the patients, still showed PFS improvement. A new potential biomarker (T–effector gene signature expression) was also evaluated in this trial, but the results as presented did not necessarily support the use of this new test for selection of patients who would benefit from this treatment above and beyond PD-L1 testing.
When to Change Standard of Care
As with many important trials, this study provided new results that could have an immediate effect on standard of care, but it also raised questions that remain unanswered:
• Does this quadruple regimen offer such a big improvement over what is available now that the additional cost and toxicity is justified?
• Is PFS an adequate endpoint for studies of this sort, or should we demand OS data before changing standard practice?
• What is the value in biomarker testing? Do the results of this study support the continued use of PD-L1 expression?
• Is cytotoxic chemotherapy capable of augmenting or inducing an immune response? Is what we see in this trial an indication that VEGF inhibition is capable of enhancing immunologic effects of checkpoint inhibitors?
Fig. 1. IMpower150 Study Design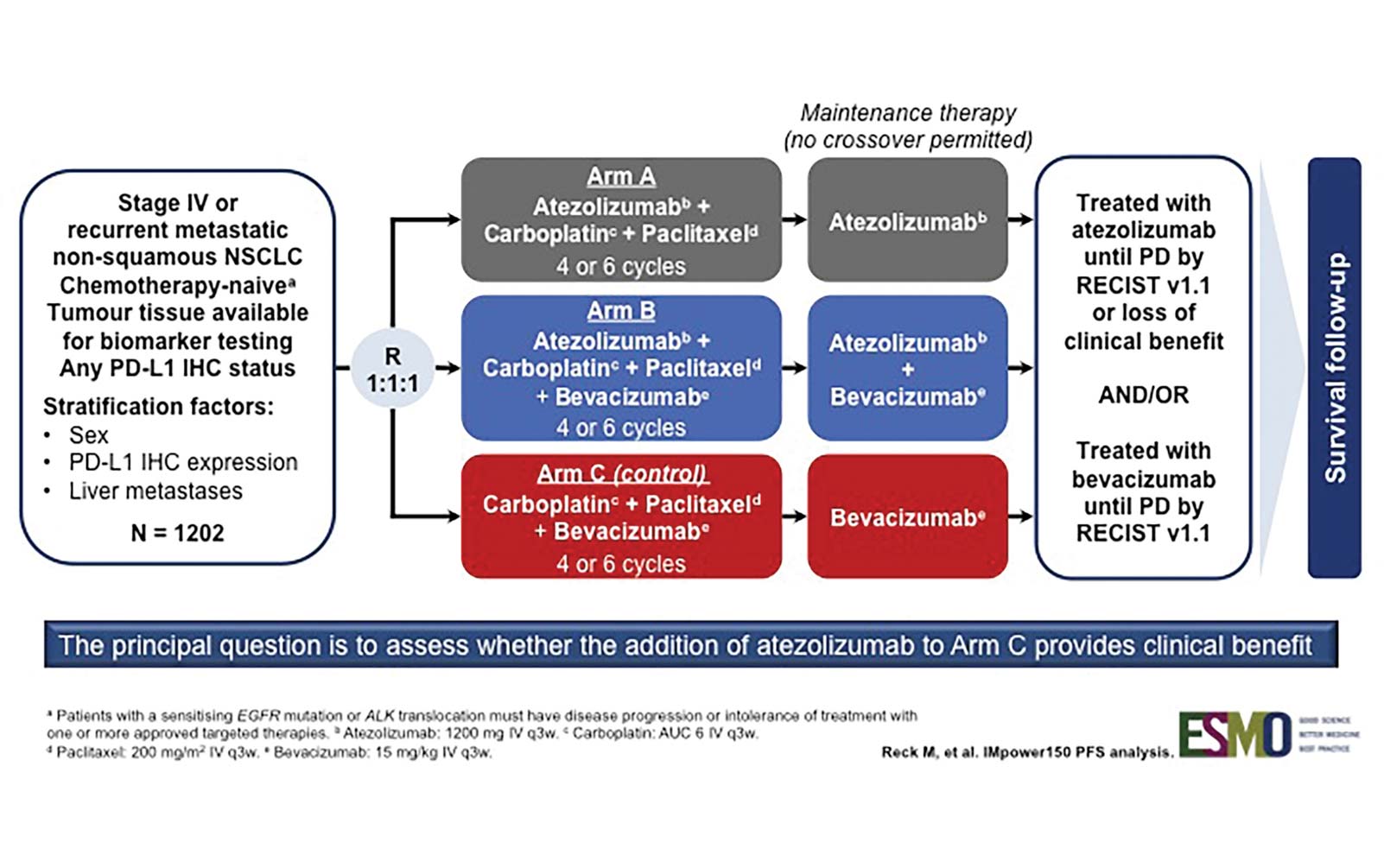 Fig. 2. INV-Assessed PFS in ITT-WT (Arm B vs. Arm C)
Fig. 2. INV-Assessed PFS in ITT-WT (Arm B vs. Arm C)
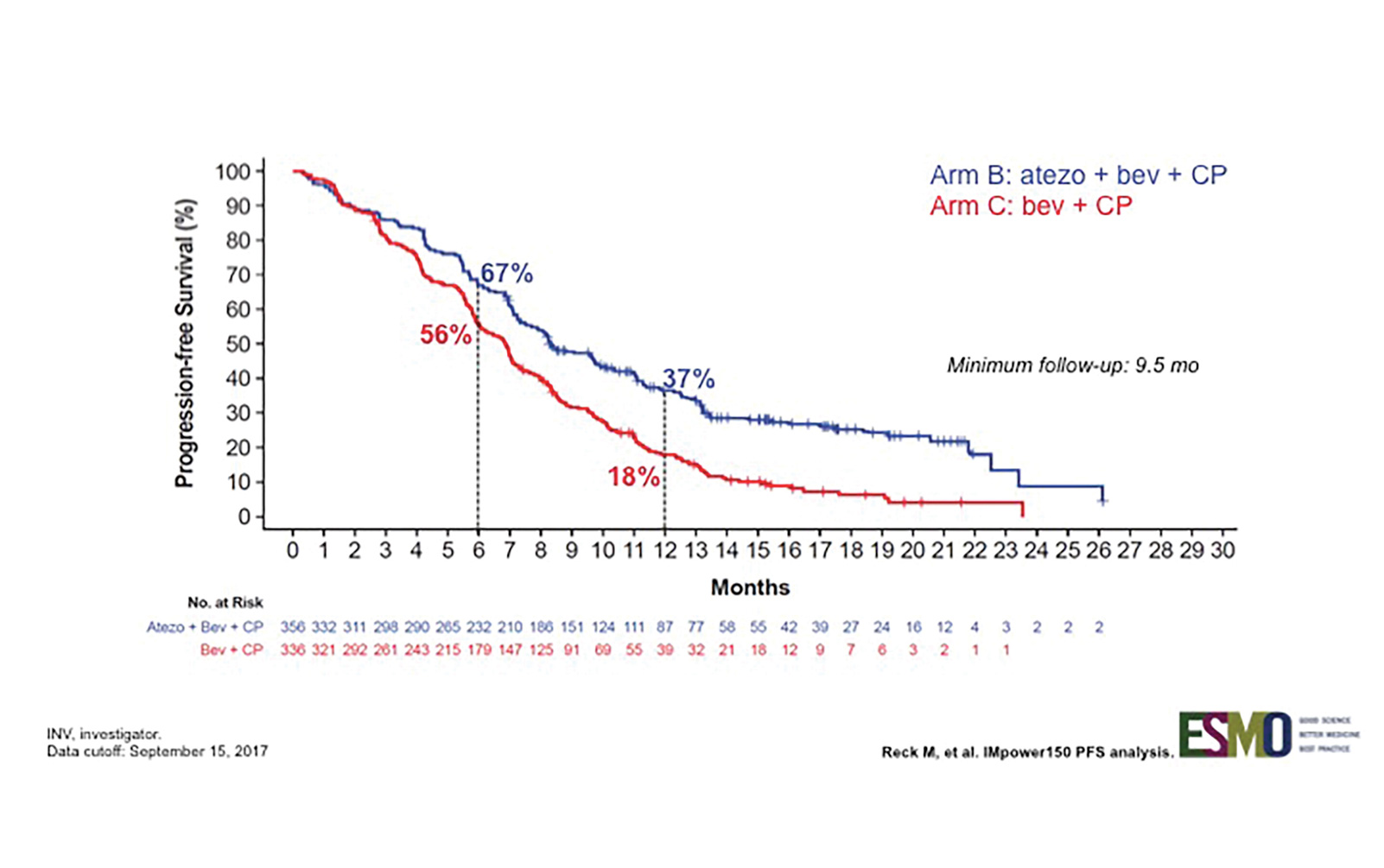
The available data so far suggest that the addition of cytotoxic chemotherapy to a checkpoint inhibitor can enhance or modulate the effects of anti–PD-1/– PD-L1 inhibitors. This is now supported by the results of KEYNOTE-021, cohort G and IMpower150. There are a number of other phase III studies using a similar approach that are expected to report soon. The bigger question is, to what extent are we able to extend our patients’ lives by offering them three- or four-drug therapies?
From my perspective, I do not believe that improved PFS alone is sufficient to consider one regimen superior to another. I still consider single-agent pembrolizumab to be the standard of care for patients with high PD-L1 expression. At this time, there is no convincing evidence that combinations are consistently improving OS in this population. For patients with lower levels of PD-L1 expression, one can argue that the higher response rates seen with these combinations justify their use, particularly for patients with high tumor burden.
Considering the fact that a sizable portion of patients with advanced lung cancer do not receive second-line therapy, it is important to use the best option upfront, but this best option is likely to be different for every individual patient—perhaps a reflection of the heterogeneity of the tumor that we are trying to defeat. ✦
About the Author: Dr. Borghaei is an Associate Professor and Chief of Thoracic Medical Oncology at Fox Chase Cancer Center.
KEYNOTE-189 Interim Results
On January 16, Merck announced that the phase III KEYNOTE-189 trial met its primary endpoints of overall survival (OS) and progression-free survival (PFS). Interim results demonstrated significantly longer OS and PFS for pembrolizumab, an anti–PD-1 therapy, in combination with pemetrexed and cisplatin or carboplatin vs. pemetrexed plus platinum chemotherapy alone. The safety profile of pembrolizumab in this combination was consistent with that previously observed. Results from KEYNOTE-189 will be presented at an upcoming medical meeting and submitted to regulatory authorities. ✦
References:
1. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1- Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-1833.
2. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-1508. 3. Presented at ESMO Immuno-Oncology Congress. Geneva, Switzerland: 2017.











