By Cynthia L. Kryder, MS and Lori L. Alexander, MTPW, ELS, MWC
Posted: December 2017
An oral abstract session at the IASLC World Conference on Lung Cancer (WCLC) 2017 drew a standing-roomonly crowd to hear updates on studies of 3 immunotherapy drugs: pembrolizumab, nivolumab, and atezolizumab, and their role in advanced non-small cell lung cancer (NSCLC). The results of these studies indicate that the benefits of PD-L1/PD-1 checkpoint inhibitors has been maintained over longer follow-up and are now extending into new settings.
Pembrolizumab
KEYNOTE-024
Julie Brahmer, MD, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, US, presented updated data from KEYNOTE-024, an international randomized phase III trial in which pembrolizumab was compared with platinum-based chemotherapy as first-line therapy for advanced NSCLC positive for PD-L1 (tumor proportion score of 50% or more) in 305 patients. The initial findings of KEYNOTE-024, after 11.2 months of follow-up, demonstrated that pembrolizumab yielded significantly longer progression-free and overall survival; the estimated 6-month overall survival was 80.2% for pembrolizumab compared with 72.4% for chemotherapy alone (P = 0.005).
Dr. Brahmer reported that, after a median follow-up of 25.2 months, pembrolizumab continues to be associated with an overall survival benefit. The median overall survival was 30.0 months for patients who received pembrolizumab compared with 14.2 months for chemotherapy (P = 0.002). She added that the improvement in overall survival with pembrolizumab was maintained despite significant crossover to pembrolizumab in the chemotherapy arm. Pembrolizumab also continued to demonstrate a favorable safety profile despite twice the duration of exposure compared to chemotherapy.
Penelope Bradbury, MB, BCh, FRACP, MD (UK), Princess Margaret Cancer Centre, University of Toronto, Canada, who discussed the abstract, noted that the findings firmly establish pembrolizumab as first-line therapy for patients with advanced NSCLC with PD-L1 expression of 50% or more. She added that further evaluation of treatment beyond progression is needed.
KEYNOTE-021, Cohort G T
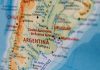
he significant benefit of pembrolizumab was also sustained in cohort G of the multicenter open-label, phase I/ II KEYNOTE-021 study, in which the efficacy and safety of pembrolizumab plus pemetrexed and carboplatin was compared with that of pemetrexed and carboplatin alone as first-line therapy for patients with stage IIIB/IV nonsquamous NSCLC, independent of PD-L1 status. Primary analysis of cohort G data (123 patients) at a minimum follow-up of 6 months (median, 10.6 months) demonstrated that pembrolizumab significantly improved the objective response rate (estimated treatment difference, 26%; P = 0.0016) and PFS. The hazard ratio for overall survival improved at a median follow-up of 14.5 months.
Corey J. Langer, MD, Perelman School of Medicine, Philadelphia, US, reported results at a median of 18.7 months of follow- up. The median PFS was significantly longer for pembrolizumab plus chemotherapy (19.0 vs. 8.9 months), and overall survival improved further (median, NR vs. 20.9 months) (Table 1).
“The incremental overall survival benefit, though not statistically significant, continues despite a high—approximately 75%—crossover rate to anti-PD-1/PD-L1 therapy in the chemotherapy-alone arm,” said Dr. Langer. He added the pembrolizumab continued to be associated with a manageable safety profile. The combination was granted accelerated approval by the US Food and Drug Administration (FDA) in May 2017.
Pembrolizumab after Local Ablative Therapy
A third study of pembrolizumab was a phase II trial to evaluate the efficacy of pembrolizumab after local ablative therapy for oligometastatic NSCLC. Joshua Bauml, MD, Assistant Professor of Medicine at the Perelman Center for Advanced Medicine, Philadelphia, US, who reported on the study, said that it has been unclear historically whether systemic therapy can provide additional benefit to local ablative therapy in the setting of metastatic disease.
Dr. Bauml reported data for 44 patients with oligometastatic NSCLC, defined as 4 sites or less. Most patients (77%) had adenocarcinoma, and most (64%) had 1 metastatic site. The most common metastatic sites were the brain (17 patients), lung (15 patients), and bone (8 patients). Metastases were metachronous in 61%.
Pembrolizumab, 200 mg every 21 days, was started within 4 to 12 weeks after local ablative therapy and was continued for 6 months, with a provision to continue for a full year in the absence of disease progression or toxicity. The median follow-up was 15 months. Dr. Bauml reported that the estimated 18-month PFS rate was 64% +/- 9% and the estimated 18-month overall survival rate was 79% +/- 8%. The most common attributable adverse events were grade 1 or 2 fatigue (34%) and arthralgia (29%). Attributable grade 3 events included 1 episode each of colitis, pneumonitis, and adrenal insufficiency.
In discussing the abstract, Dr. Bradbury said that these preliminary data indicate that incorporating a PD-1 inhibitor with local ablative therapy appears feasible. Given the nature of the grade 3 adverse events, she posed the question of whether toxicity was associated with the location of ablative therapy.
Atezolizumab
BIRCH
Enric Carcereny Costa, MD, Catalan Institute of Oncology Badalona, Germans Trias i Pujol Hospital Badalona, Barcelona, Spain, presented updated survival data from BIRCH, a phase II trial of atezolizumab monotherapy in PD-L1- selected patients with locally advanced or metastatic NSCLC. Dr. Costa highlighted data for 138 patients who received first-line treatment with atezolizumab. All patients had tumors that expressed PD-L1 at a level of at least 5%; a subgroup of patients had expression levels of 50% or more.
In the primary analyses, atezolizumab monotherapy showed meaningful and durable clinical benefit across multiple lines of therapy (first-line, second-line, and beyond). Dr. Costa reported that after nearly 3 years of follow-up (median 34.3 months), the apparent survival benefit remained durable. In addition, efficacy was demonstrated in patients with either wild-type or mutated tumors (EGFR or KRAS). Median overall survival was 26.9 months for 65 patients who had PD-L1 expression of 50% or more and 24.0 months for all patients; the corresponding investigator-assessed objective response rates were 35% and 26%. Among evaluable patients, the overall response rate was 31% for patients with EGFR-mutant tumors (4 of 13 patients) vs. 23% for patients with wild-type EGFR (24 of 103 patients), and 31% for KRASmutant tumors (10 of 32 patients) vs 24% for wild-type KRAS (16 of 66 patients). No new safety signals were observed.
Dr. Costa noted that data from ongoing phase III trials such as the IMpower110 study will further define the role of atezolizumab monotherapy as first-line therapy for this group of patients. Discussant Martin Schuler, MD, University Hospital Essen, Essen, Germany, remarked that pembrolizumab remains the standard of care for people with NSCLC with high PD-L1 expression (tumor proportion score of at least 50%), excluding EGFR-mutant and ALKrearranged lung cancers.
OAK
In the randomized phase III OAK trial, atezolizumab demonstrated superior survival compared with docetaxel in patients with NSCLC who had received 1 or 2 previous lines of chemotherapy, independent of PD-L1 status. Miyako Satouchi, MD, PhD, Hyogo Cancer Center, Akashi, Japan, reported on the characteristics of the long-term survivors from the OAK primary population (850 patients), defined as patients who lived 2 years or longer after randomization.
The survival rate was higher for the atezolizumab arm compared to the docetaxel arm (Table 2). After a minimum follow-up of 26 months, the atezolizumab arm included more longterm survivors than the docetaxel arm. The group of long-term survivors who received atezolizumab were enriched for nonsquamous histology and high PD-L1- expressing tumors, but 40% of patients had tumors with low or no PD-L1 expression. The objective response rate for atezolizumab long-term survivors was higher than those who did not live 2 years, but 25 long-term survivors (21%) had progressive disease as their initial disease assessment.
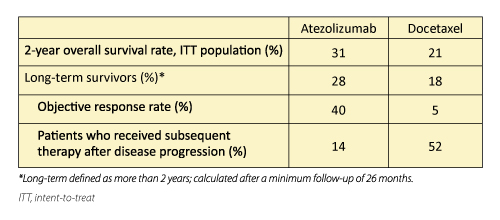
Dr. Satouchi noted that long-term survival with atezolizumab was associated with extended treatment duration and was not limited to patients who had a radiographic response. Median treatment exposure for atezolizumab long-term survivors was 18 months. Dr. Bradbury indicated that treatment with atezolizumab could continue beyond progression if the investigator believed the patient was deriving a clinical benefi t. She noted that progressive disease, according to RECIST v1.1 criteria, encompasses several diff erent scenarios and the question of whether to treat after progression remains unanswered.
Nivolumab
CheckMate-012
Rosalyn Juergens, MD, PhD, Juravinski Cancer Centre at McMaster University, Hamilton, Canada, presented a 3-year update of the safety and effi cacy of fi rstline nivolumab combined with platinumbased doublet chemotherapy in the randomized phase I CheckMate-012 study. Fift y-six chemotherapy-naive patients with stage IIIB/IV NSCLC were randomly assigned, based on histology, to 3 cohorts combining nivolumab with platinum- based doublet chemotherapy regimens: nivolumab + pemetrexed–cisplatin; nivolumab + paclitaxel–carboplatin; and nivolumab + gemcitabine–cisplatin.
Nivolumab plus chemotherapy had a tolerable and manageable safety profile, and no new safety signals were observed aft er a minimum follow-up of 46 months.
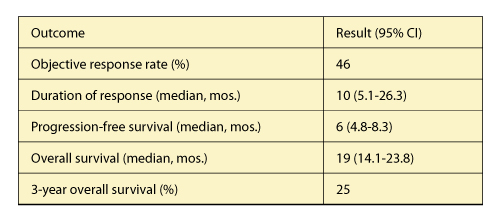
For all patients, objective response rate and overall survival were similar, irrespective of tumor PD-L1 expression (Table 3). Th ree-year survivors included patients with PD-L1 expression of less than 1% and never-smokers; 69% of 3-year survivors received subsequent systemic therapy.
In discussing the abstract, Dr. Schuler noted that although CheckMate-012 provides interesting clinical signals with regard to immunotherapy-chemotherapy combinations in unselected patients, single-arm studies or small randomized phase II trials should not be the basis for changes in the standard of care in unselected NSCLC populations. Multiple suffi ciently powered, pivotal phase III studies are underway; he eagerly awaits the results. Figure 1. IFCT-1502 Clinivo Overall Survival and Progression-Free Survival: Nivolumab-treated Patients
IFCT-1502 CLINIVO
Real-life experience with nivolumab in 902 patients with advanced NSCLC was the topic of the presentation by Nicolas Girard, MD, Hospices Civils de Lyon, Lyon, France. Dr. Girard reported the results of a retrospective medical-record analysis of patients with squamous (317 patients) and nonsquamous (585 patients) stage IIIB/IV NSCLC who received at least 1 dose of nivolumab. Endpoints included overall survival, best response, maximum toxicity of nivolumab, and predictors of response.
Median age was 64 years; 782 patients (86%) were smokers and 197 (22%) had brain metastases. Nivolumab was administered as second-, third-, or fourth-line treatment and beyond to 27%, 32%, and 41% of patients, respectively. Th e objective response rate for nivolumab was 19%, with stable disease in 36%, and progressive disease in 45% of patients; 1% were not evaluable. Toxicities occurred in 313 (35%) patients treated with nivolumab, including grade 3 or higher events in 11% of patients. Median overall survival and progression-free survival were also calculated (Figure 1). Prognostic factors of overall survival included performance status and the presence of at least 2 brain metastases, whereas sex, age at initiation of nivolumab, smoking history, TNM stage, and histology were not associated with overall survival.
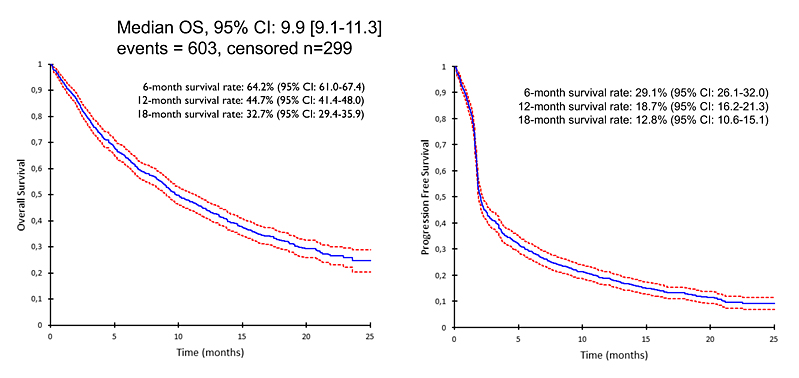
Post‐nivolumab treatment was given to 426 (47%) patients;319 of these patients (75%) receiving systemic therapy. Patients with a performance status of 0 or 1, those who received 1 or 2 prior lines of therapy, and those without brain metastases at the time of nivolumab initiation were more likely to receive treatment aft er nivolumab. Best response to the fi rst post-nivolumab treatment was objective response in 16%, stable disease in 42%, and progressive disease in 42% of patients.
Dr. Girard concluded that these effi – cacy and safety results for nivolumab are commensurate with available data on nivolumab and other immunotherapy agents. He noted that post-nivolumab treatment may be delivered to many patients, including those with a performance status of 2 or worse, or brain metastases, with highly variable effi cacy and impact on overall survival. ✦