By Maria Antonia Velez, MD; Nanruoyi Zhou, BA; Brian Henick, MD; and Aaron Lisberg, MD
Posted: August 19, 2020
Immune checkpoint inhibitors (CPIs) have revolutionized the treatment of metastatic NSCLC. However, approximately 20% to 30% of patients with metastatic NSCLC have an oncogene-driven disease, for which targeted therapies with mutation-specific tyrosine kinase inhibitors (TKIs) are the first-line therapy.1 There is growing data to suggest that patients with oncogene-driven disease, particularly those with minimal or no smoking history, experience limited benefit from CPIs, as well as disproportionately higher toxicity.2-5 Nevertheless, it is important to consider that molecular testing is often delayed by a median of 3 weeks after initial diagnosis, which can be both anxiety provoking as well as clinically detrimental to patients with metastatic NSCLC who could potentially benefit from first-line CPIs in the absence of molecular data.6 Thus, identifying the appropriate timing for the introduction of CPIs relative to targeted agents in patients who are candidates for systemic therapy largely depends on weighing clinicopathologic features such as the smoking history, tumor histology, and PD-L1 proportion score. Other factors to consider include the rate of disease progression and the psychosocial circumstances of the patient. It is particularly important to identify patients with rapidly progressive disease, defined as disease trajectory that is likely to lead to either an oncologic emergency or functional decline, limiting additional therapy within 6 weeks.7
When a patient is initially diagnosed with metastatic NSCLC, it is important to identify the presence of targetable mutations as well as PD-L1 status before the initiation of a CPI. This is because patients with EGFR mutations or ALK/ROS1 rearrangements show limited benefit with CPIs even in the presence of PD-L1 tumor expression of 50% or higher.2,4,8 Results from KEYNOTE-189 were met with great excitement, as they demonstrated that patients with advanced adenocarcinoma benefited from frontline chemoimmunotherapy regardless of PD-L1 status.9 However, this study did not include patients with EGFR mutations or ALK rearrangements and, thus, extrapolating these outcomes to the EGFR/ALK (+) patient population is not appropriate. Similarly, IMpower150 evaluated chemoimmunotherapy combination strategies in patients with advanced adenocarcinoma, including a subgroup of patients with EGFR mutations and ALK rearrangements.10 This subgroup of patients did benefit from a combination regimen of chemoimmunotherapy with VEGF-directed therapy, but at the cost of high rates of grade 3 and 4 adverse events, and the patient population evaluated was limited in number.10In addition, the majority of those with EGFR mutations or ALK rearrangements had previously received TKIs.
Studies evaluating a combination strategy of TKIs plus immunotherapy have often resulted in high, if not untoward, toxicity rates.11-13 Further, increased immunotherapy toxicity has been observed in patients receiving TKI therapy, even when the two classes of therapy are given sequentially.2,5,14 This suggests that patients with EGFR/ALK/ROS1 alterations should be spared from these high toxicity rates in the frontline setting,15 and that identifying the right first line treatment is critical. When a targetable molecular alteration does not exist, then treatment decisions should be based on PD-L1 status using 50% as the cutoff, where patients who fall below the cutoff should receive chemoimmunotherapy and patients above the cutoff can receive CPI monotherapy, unless they exhibit rapidly progressing disease.9,16
Patients with molecular alterations in BRAF, HER2, nTRK, and MET pose a unique challenge with respect to timing of CPI introduction, as data on the use of CPIs in these populations are scant. A recent study suggested that patients with BRAF mutations have high levels of PD-L1 expression and may benefit from CPIs.17 Furthermore, a retrospective study of patients with advanced NSCLC receiving CPI monotherapy showed an OS of 20.3 months for patients with BRAF mutations and 18.4 months for patients with MET mutations, compared to 10 months for patients with EGFR mutations and 3.6 months for patients with ALK rearrangements.18 In a small cohort of patients with HER2-mutated NSCLCs, three out of 26 had responses but poor durability.19 Some case reports and case series have suggested a role for CPIs in patients with MET mutations whose disease had progressed on crizotinib.20 Insufficient data exist on the use of CPIs in patients with MET amplifications. Similarly, there are limited data on the role of CPIs for patients with nTRK mutations.
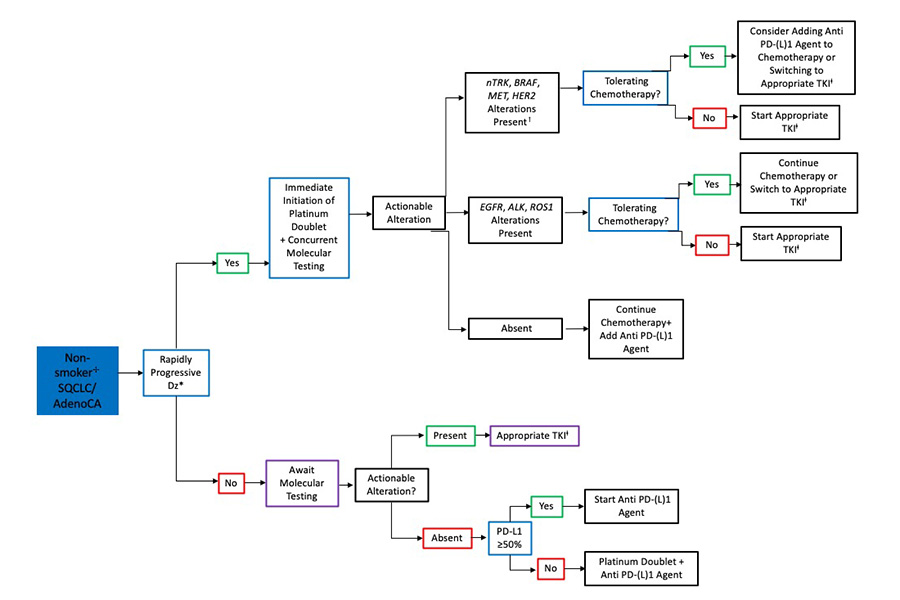
The approach to determining the appropriate timing of CPIs with respect to molecular testing in patients with adenocarcinoma (AC) and in never-smokers with metastatic squamous cell lung cancer (SQCLC) is shown in Fig. 1. Of note, a positive smoking history is defined as a minimum of 100 cigarettes over a lifetime.21 In all patients with AC and in non-smokers with SQCLC, molecular testing is recommended prior to CPI initiation but does not necessarily need to delay treatment initiation. In situations where treatment is urgently required such as rapidly progressive disease, physician’s gestalt, or patient’s demand, we recommend the initiation of chemotherapy alone or in combination with bevacizumab while awaiting molecular data. If an EGFR, ALK, or ROS1 alteration is identified, providers have the option of continuing chemotherapy alone or switching to a TKI. In patients with MET amplification, MET exon 14 skipping mutation, or HER2, nTRK, or BRAF alterations, the introduction of a PD-(L)1 agent during future chemotherapy cycles is appropriate, as is switching to the appropriate TKI.

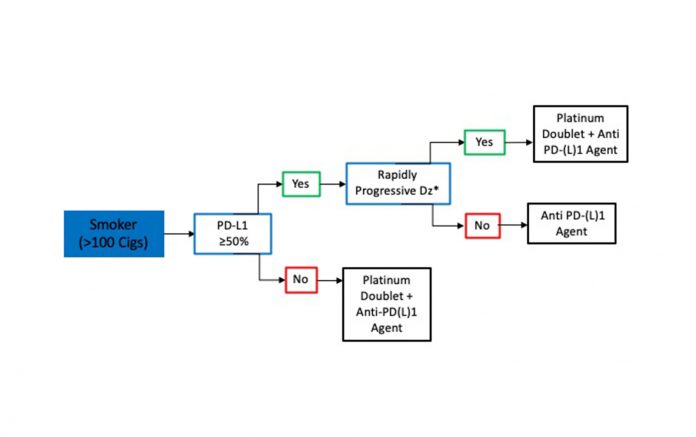
In smokers with metastatic SQCLC, treatment decisions are based on PD-L1 status, and a delay in CPI initiation for molecular testing is not required (Fig. 2). Thus, for patients with metastatic SQCLC who have a smoking history, a PD-L1 tumor score of 50% or greater and no evidence of rapidly progressive disease, treatment with pembrolizumab monotherapy can be started without testing for targetable mutations since the likelihood of an actionable molecular aberration is quite low.16 However, in smokers with rapidly progressive SQCLC or with a PD-L1 score lower than 50%, we favor administration of a CPI in combination with platinum-doublet chemotherapy.7,22
In conclusion, to determine the optimal timing for CPIs with respect to molecular data in NSCLC, it is important to determine the patient’s smoking history, the rate of disease progression, and tumor histology (Figs. 1 and 2). These characteristics can help differentiate the patients for whom it is imperative to await molecular testing results prior to initiation of CPIs from those who can be started on CPIs immediately.
About the Authors: Dr. Velez is with the Department of Internal Medicine, University of Pittsburgh Medical Center. Dr. Zhou is with UCLA David Geffen School of Medicine. Dr. Henick is with Columbia University Medical Center. Dr. Lisberg is with UCLA David Geffen School of Medicine.
References:
1. Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415-1426.
2. Lisberg A, Cummings A, Goldman JW, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J Thorac Oncol. 2018;13(8):1138-1145.
3. Lisberg A, Garon EB. The Italian Nivolumab Expanded Access Program Confirms the Limitations of Single-Agent PD-1 Inhibition in EGFR-Mutant and Never-Smoking Patients with NSCLC. J Thorac Oncol. 2018;13(8):1058-1059.
4. Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol. 2017;12(2):403-407.
5. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(8):1112-1115.
6. Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non-small cell lung cancer: optimizing the diagnostic journey. Transl Lung Cancer Res. 2019;8(3):286-301.
7. Lisberg A, Garon EB. Does Platinum-Based Chemotherapy Still Have a Role in First-Line Treatment of Advanced Non-Small-Cell Lung Cancer? J Clin Oncol. 2019;37(7):529-536.
8. Mazieres J, Drilon AE, Mhanna L, et al. Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget). J Clin Oncol. 2018;36(15_suppl):9010-9010.
9. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078-2092.
10. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
11. Liang H, Liu X, Wang M. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther. 2018;11:6189-6196.
12. Gettinger S, Politi K. PD-1 Axis Inhibitors in EGFR- and ALK-Driven Lung Cancer: Lost Cause? Clinic Cancer Res. 2016;22(18):4539-4541.
13. Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol. 2016;11(4, Supplement):S115.
14. Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30(5):839-844.
15. Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22(18):4585-4593.
16. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823-1833.
17. Dudnik E, Peled N, Nechushtan H, et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J Thorac Oncol. 2018;13(8):1128-1137.
18. Arulananda S, Mitchell P. BRAF Mutations-A Good News Story for Immune Checkpoint Inhibitors in Oncogene-Addicted NSCLC? J Thorac Oncol. 2018;13(8):1055-1057.
19. Lai W-CV, Feldman DL, Buonocore DJ, et al. PD-L1 expression, tumor mutation burden and response to immune checkpoint blockade in patients with HER2-mutant lung cancers. J Clin Oncol. 2018;36(15_suppl):9060-9060.
20. Mayenga M, Monnet I, Massiani M, et al. MA03.09 Dramatic Responses to Immune Checkpoint Inhibitors in MET Exon 14 Skipping Mutation (METex14mut) Non Small Cell Lung Cancers. J Thorac Oncol. 2019;14(10):S259.
21. Centers of Disease Control and Prevention. Glossary. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm. Published 2017. Updated August 29, 2017. Accessed March 15, 2020.
22. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2040-2051.