Median OS was longer in patients with epithelioid histology (21.2 months) than in those with non-epitheliod histology (12.1 months).
Ceresoli et al, Lancet Oncol 2019
By Giovanni Luca Ceresoli, MD
Posted: June 24, 2020

Tumor-treating fields (TTFields) are a noninvasive, regional treatment based on the delivery of low-intensity alternating electric fields by transducer arrays applied to the patient’s skin surrounding the tumor.1 TTFields extend survival in glioblastoma and are U.S. Food and Drug Administration (FDA) approved for newly diagnosed and recurrent glioblastoma. The STELLAR trial2 was a prospective, phase II, single-arm pilot study in which patients with unresectable malignant pleural mesothelioma (MPM) received treatment with TTFields, delivered continuously by the NovoTTF-100L system at a frequency of 150 kHz, for a planned daily duration of at least 18 hours/day. Chemotherapy with intravenous pemetrexed and platinum (cisplatin or carboplatin, according to the investigator’s choice) was administered concomitantly at standard doses, for up to 6 cycles. Patients whose disease did not progress after the combined TTFields/chemotherapy regimen received maintenance TTFields alone until unacceptable toxicity, progression, or patient refusal intervened. The primary endpoint of the trial was OS, and the secondary endpoints were PFS, overall response rate (ORR) according to modified RECIST criteria3 per investigator’s assessment, 1- and 2-year survival, and safety.
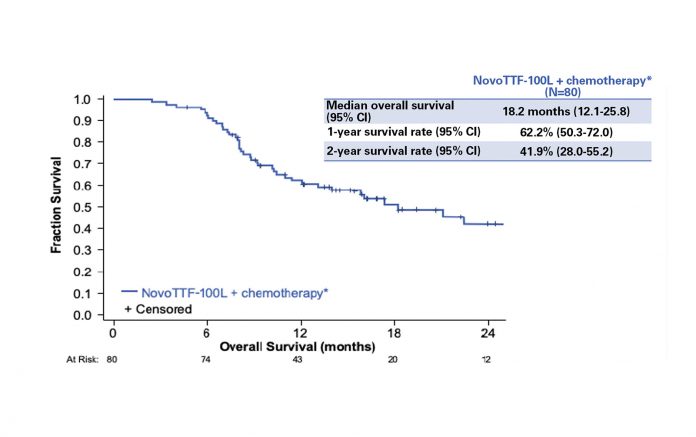
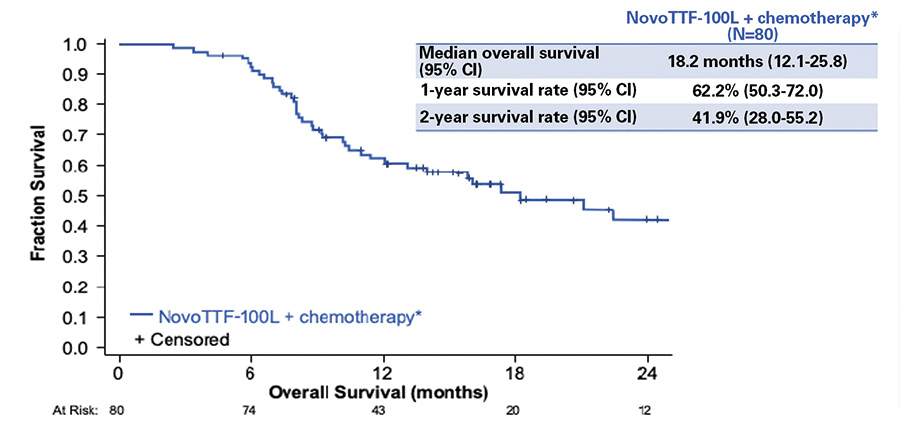
Eighty patients were enrolled and included in the final analysis. The study results showed that TTFields applied to the thorax in combination with pemetrexed and platinum were an active and safe option in the frontline treatment of unresectable MPM. Median OS was 18.2 months, with 1-year and 2-year OS of 62% and 42%, respectively (Fig.1). Patients with epithelioid subtype had a median OS of 21.2 months versus 12.1 months for those with nonepithelioid tumors. Overall median PFS was 7.6 months: 8.3 months in patients with epithelioid tumors versus 6.5 months in patients with non-epithelioid tumors. A partial response was observed in 29 (40%) of 72 evaluable patients. No increase in systemic toxicity was reported with the addition of TTFields to standard chemotherapy; the only adverse event related to the device was skin irritation underneath the transducer arrays. Grade 1-2 dermatologic adverse events (dAEs) were observed in 66% of patients; only 5% had grade 3 skin toxicity, leading to temporary or permanent interruption of the treatment. In the majority of cases, dAEs were managed with topical corticosteroids, short treatment breaks, and a regular shifting of 1-2 cm at each array change.
Median OS was longer in patients with epithelioid histology (21.2 months) than in those with non-epitheliod
histology (12.1 months).
Ceresoli et al, Lancet Oncol 2019
As with any single-arm phase II study, the STELLAR trial has obvious limitations, suggesting that further data are needed before there can be widespread implementation of this therapeutic strategy in clinical practice. However, sample size was relatively large for a trial in this rare malignancy, and patient baseline characteristics, including ECOG performance status, were in line with other recent MPM studies, such as the MAPS4 and LUME-Meso5 trials. Of note, 26% of patients on the STELLAR trial had a non-epithelioid tumor, a recognized negative prognostic factor in MPM. The primary outcome measure of the study was OS, which, as of 2020, should be the standard endpoint for trials in advanced MPM, as stated by a recent consensus report.6 Endpoints assessed radiographically, such as ORR and PFS, are challenging in this disease, especially if a central radiographic review is not planned. Of note, OS of patients on the STELLAR trial was likely not biased by post-study treatments (Fig. 2).
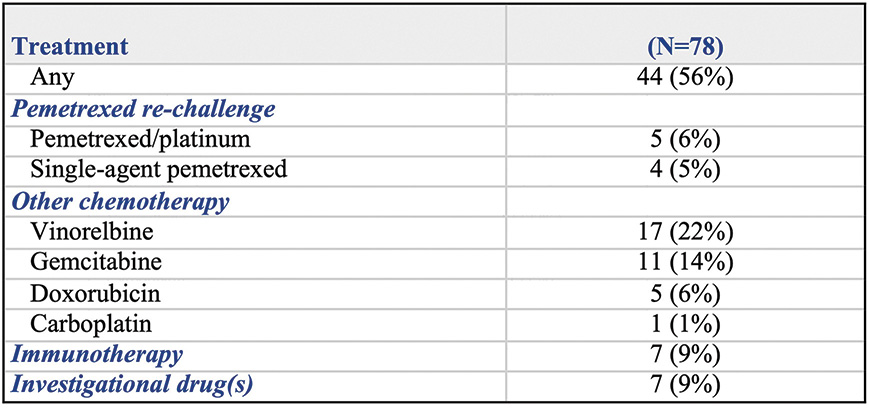
Patients could have more than one subsequent anticancer therapy. Data were not available in 2 patients.
Ceresoli et al, Lancet Oncol 2019
Based on the results of the STELLAR trial, the FDA has approved the use of TTFields with pemetrexed/platinum for the treatment of unresectable locally advanced or metastatic MPM, under the Humanitarian Device Exemption pathway.7 This is the first FDA approval to be granted for mesothelioma since 2004.
Areas of Improvement
TTFields act mainly by disrupting the mitotic spindle, but the molecular determinants that affect tumor sensitivity are currently unexplored. The treatment continues to be studied both preclinically and clinically in MPM as well as in other solid tumors, such as lung, pancreatic, ovarian, liver, and gastric cancers. The recent availability of an animal model using this technology8 could contribute to the identification of biomarkers predictive of benefit.
dAEs were the only toxicities directly related to TTFields in the STELLAR trial. Although generally low-grade, easily manageable events, dAEs require the physician’s attention to ensure they do not lead to treatment breaks (Fig. 3). They can occur as a result of a number of different stimuli, including repetitive mechanical trauma (as in the application and removal of the arrays and in shaving) and inflammation (from the adhesive patch or from the hydrogel covering the ceramic discs).9 A recent metaanalysis10 evaluated the adverse events observed in 192 patients enrolled in four pilot clinical studies addressing different cancers, where TTFields in combination with chemotherapy were delivered to the torso. Consistent with STELLAR, dAEs occurred in 58% of cases; low-grade dermatitis was reported in 53% and high-grade in 6%. Low-grade pruritus was reported in 9%. There were no other significant TTFields–related AEs.
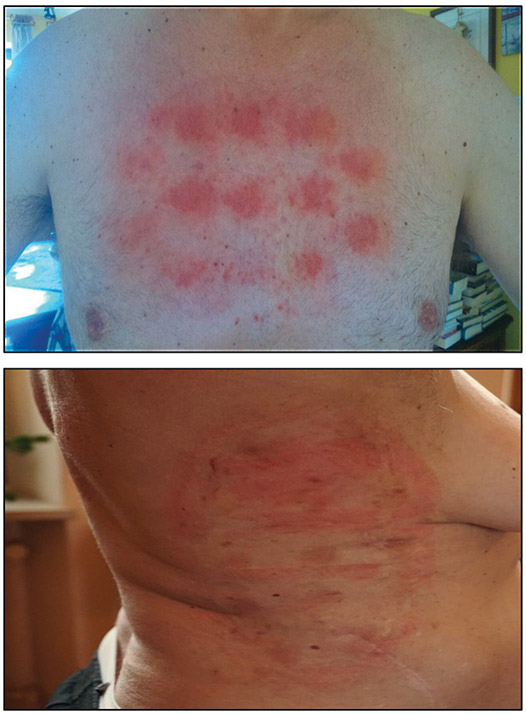
Each set of arrays should be changed at least every 3-4 days. More frequent array changes may be required in some patients (e.g., during warmer weather or after intense physical activity.)
Careful removal of arrays (taking approximately 60 seconds to remove each array). Excessive force should be avoided. Use mineral (baby) oil on the edge of the arrays during removal.
Shift the arrays of 1-2 cm from the previous position at each change. The skin must be completely dry before applying a new set of arrays.
If there are signs of dermatitis, early treatment with a highpotency topical corticosteroid is recommended (e.g., clobetasol 0.05%, betamethasone 0.05%). When the epidermal barrier is compromised (erosions) or when there are signs of infection, topical antibiotics are recommended.
Lacouture et al, Semin Oncol 2014
Quality-of-life (QoL) data assessment was not planned in the STELLAR trial. A secondary analysis of the phase III EF-14 trial in newly diagnosed glioblastoma showed no adverse effect on QoL for the addition of TTFields to standard chemotherapy, except for more itchy skin, an expected consequence from the transducer arrays.11 Similarly, the use of the device is not expected to lead to QoL deterioration in patients with MPM. At the same time, increased compliance with TTFields therapy was an independent prognostic for survival in the glioblastoma trial.12 Proactive skin care and the availability of a smaller and lighter device may be valuable strategies to improve QoL and compliance to treatment (and ultimately outcomes) in patients with MPM. ✦
About the Author: Dr. Ceresoli is with the Department of Oncology, Cliniche Humanitas Gavazzeni, Bergamo, Italy.
References:
1. Mun EJ, Babiker HM, Weinberg U, et al. Tumor Treating Fields: A fourth modality in cancer treatment. Clin Cancer Res. 2018;24:266-275.
2. Ceresoli GL, Aerts JG, Dziadziuszko R, et al. Tumor Treating Fields in combination with pemetrexed and cisplatin/carboplatin as first line treatment for unresectable malignant pleural mesothelioma: results of the STELLAR multicenter, single arm, prospective phase 2 trial. Lancet Oncol. 2019;20:1702-1709.
3. Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257-260.
4. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405-1414.
5. Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2019;7(7):569-580.
6. Tsao AS, Lindwasser OV, Adjei AA, et al. Current and future management of malignant mesothelioma: a consensus report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol. 2018;13:1655-1667.
7. NovoTTFTM – 100L System – H180002. U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/recently-approved-devices/novottftm-100l-system-h180002. Published May 28, 2019. Accessed January 12, 2020.
8. Voloshin T, Kaynan N, Davidi S, et al. Tumor-Treating Fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020 Mar 6. [Epub ahead of print].
9. Lacouture ME, Davis ME, Elzinga G, et al. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014;41(Suppl 4):S1-S14.
10. Ceresoli GL, Vergote I, Rivera F, Pless M. Safety of Tumor Treating Fields delivery to the torso: pooled analysis from TTFields clinical trials (Abstract 8215). Proceedings of the 2019 Annual Meeting of the American Association for Cancer Research. 2019;79(13 Suppl.).
11. Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495-504.
12. Toms SA, Kim CY, Nicholas G, Ram Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neuro Oncol. 2019;141(2):467-473.
Related Articles:
TTFields in Mesothelioma (The STELLAR Trial): More Stars Needed in the Constellation