By Paul Baas, MD, PhD, and Cornedine J. de Gooijer, MD
Posted: June 24, 2020
Recently, a manuscript by Ceresoli et al. was published in The Lancet Oncology.1 The study states that the use of tumortreating fields (TTFields) is a major improvement in the treatment of patients with mesothelioma compared to previous standards.2 Herein, we take a closer look at the study.
Background of TTFields Application
Previous experimental studies have indicated that the use of low-frequency electric fields may disrupt tumor progression through the mitotic spindle during mitosis. In addition, heat is produced that may augment a cytotoxic reaction.2 However, cells were only tested in vitro, and no clear explanation is given as to why 150 kHz is the optimal frequency.
The device has been approved based on the lack of significant toxicity and electronic safety of the apparatus.3 The U.S. Food and Drug Administration, however, did report that although the results of the STELLAR study are within the range (of reported PFS and OS) and show improvement, in the absence of a control group receiving chemotherapy alone, statistical significance was not determined.
It is a well-known scientific observation that single-armed studies nearly always tend to overestimate outcomes observed in randomized trials or in the real world. The results of STELLAR suggest similarity with the data obtained from the nintedanib study where the (randomized) phase II single-armed study suggested benefit, but which failed in the phase III setting in mesothelioma.4,5
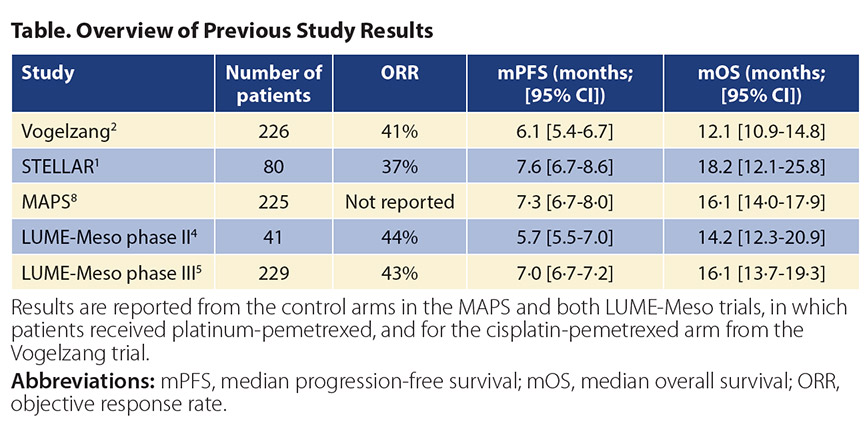
Not Written in the Stars (Yet) The STELLAR trial reported a median OS (primary endpoint) of 18.2 months (95% CI [12.1, 25.8]). The study looked for an OS of 17.6 months, an increase of 5.5 months in median OS compared to the results obtained for combination cisplatin and pemetrexed in the registration study in 2003.2 However, these results should be placed in the context of recent trials in mesothelioma.6 The Table shows the objective response rate, PFS, and OS in previous randomized trials in patients with malignant mesothelioma treated with platinum-pemetrexed. The confidence intervals observed for TTFields in combination with standard chemotherapy in the STELLAR trial overlap with historical data. Although the authors attributed the lack of benefit for angioinhibitory strategies in the MAPS and LUME-Meso trials to a patient population with a worse prognosis (more patients with sarcomatoid mesothelioma), other prognostic factors (like platelet count and lactate dehydrogenase) were not reported. This underscores the need for randomized studies to reveal the true added benefit of TTFields in mesothelioma.
The monthly costs of TTFields ($21,000 in 2016) and the effect on quality of life need to be justified based on phase III data. TTFields were used continuously during the four cycles of platinum-pemetrexed therapy and as maintenance therapy until progression. Patients received a median of eight cycles of TTFields, hence a median 5.5 months of continual use of this device. The effect on quality of life for patients and relatives should not be underestimated.7 The TTFields device, applied to the thorax, is recommended to be used for 18 hours per day (also at night). In 66% of patients, a local reaction to the skin at the site of the medical device was observed.1
Based on the results of this study, we conclude that the TTFields approach may hold some promise, but there is a clear lack of prospective phase III evidence. A randomized study is of paramount importance, and more insight into its working mechanisms is needed. Differences in pathology and performance status of the patients enrolled on this study compared to populations on other trials and historic controls can account for potential spread in outcome. Finally, we need to focus on translational research factors to support a role for this device. ✦
About the Authors: Dr. Baas is chief of Department of Thoracic Oncology, Netherlands Cancer Institute, Amsterdam, the Netherlands. Dr. de Gooijer is with the Department of Thoracic Oncology, Netherlands Cancer Institute, Amsterdam, the Netherlands.
References:
1. Ceresoli GL, Aerts JG, Dziadziuszko R, et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 2019;20(12):1702-1709.
2. Vogelzang NJ, Rusthoven JJ, Symanowski, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21;2636-2644.
3. Administration FaD. HDE H180002 FDA Summary of Safety and Probable Benefit. https://www.accessdata.fda.gov/cdrh_docs/pdf11/H110002b.pdf. Accessed January 14, 2020.
4. Grosso F, Steele N, Novello S, et al. Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial. J Clin Oncol. 2017;35(31):3591-3600.
5. Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2019;7(7):569-80.
6. de Gooijer CJ, Burgers JA. Tumour Treating Fields for mesothelioma. Lancet Oncol. 2020;21(1): e9.
7. Kwan K, Schneider JR, Boockvar JA. Quality of Life in Patients With Glioblastoma Treated With Tumor-Treating Fields. JAMA. 2018;319(17):1822-1823.
8. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet (London, England). 2016;387(10026):1405-1414.
Related Articles:
TTFields in Unresectable MPM: STELLAR Results in Practice