By Tetsuya Mitsudomi, MD
Posted: April 16, 2020
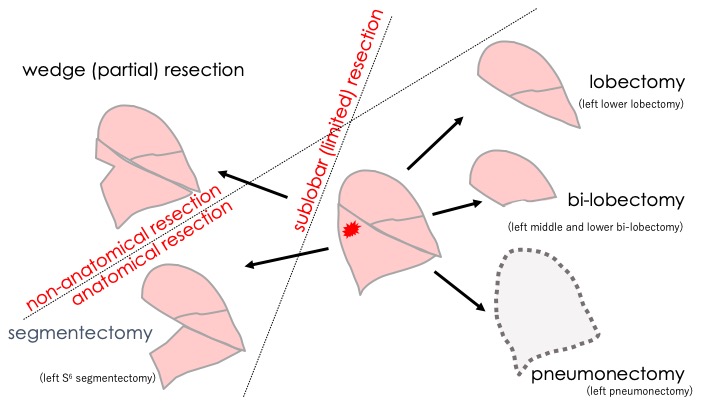
The general principle of surgery for cancer of any organ is to remove the entire tumor with adequate margins of normal tissue along with regional lymph nodes that may have the potential for metastatic spread, even if preoperative imaging studies do not indicate any signs of metastases. The lung is composed of lobes, and each lobe is composed of segments. Therefore, there are several potential operative procedures for lung cancer, depending on the amount of lung parenchyma to be removed (Fig. 1). Segmentectomies and wedge resections (also called partial resections) are often referred to as sublobar or limited resections. Wedge resections are also referred to as nonanatomical resections, in contrast to other resections that remove at least one segment of the lung taking the bronchovascular anatomy into consideration.
Pneumonectomy to Lobectomy as a Standard Procedure
The first long-term survival after lung cancer surgery was achieved in 1933 by Graham and Singer,1 who performed a left pneumonectomy for a 48-year-old male gynecologist. The patient survived for 30 years after surgery. In 1950, Churchill2 at the Massachusetts General Hospital reported that 30-day mortality for pneumonectomies and lobectomies for lung cancer during 1933-1950 was 23% and 14%, respectively, with 5-year survival rates of 12% and 19%, respectively, suggesting that lobectomies might be safer with better long-term survival compared with pneumonectomies. In the 1950s, pneumonectomy, however, was regarded as a standard of care when big surgery was assumed to yield maximal chance of cure, as exemplified by Halsted’s radical mastectomy. In 1962, Shimkin et al.,3 at the biometry branch of the National Cancer Institute, retrospectively compared the survival of 116 cases of lung cancer treated by lobectomy with 402 cases treated by pneumonectomy. They reported that lobectomies resulted in at least equivalent prognosis for either those with localized or non-localized disease.3 In accordance with these data, lobectomies gradually became the standard procedure. For example, at the Massachusetts General Hospital, the percentages of pneumonectomies in total lung resection decreased from 57% in 1931-1940 to 11% in 1961-1970.4 More recently, it was reported that of 79,953 patients who underwent pneumonectomy or lobectomy for lung cancer between 2004 and 2013, only 5% had pneumonectomy according to the National Cancer Database.4a The 5-year unadjusted survival rates were 46% and 60% for pneumonectomy and lobectomy, respectively.4a In Japan, pneumonectomy only accounted for 1.8% of 18,073 pulmonary resections for lung cancer performed in 2010.4b
Sublobar Resections as a Therapeutic Option
Many patients with lung cancer do not tolerate lobectomies because they are often older with impaired physiologic reserves due to coexisting morbidities (e.g., chronic obstructive lung disease) which is often related to tobacco smoking. In addition, when the tumor is very small, it might not be necessary to remove an entire lobe. Therefore, it is tempting to consider sublobar resections for selected patients. Jensik et al.5 reported the early results of segmentectomies for lung cancer with a 5-year survival rate of 56% in 69 patients undergoing curative resection. With the advent of the CT scanner in the late 1970s, it became possible to detect smaller lung cancers, which further promoted the wave toward these tissue-sparing resections.
The Lung Cancer Study Group (LCSG), led by Robert J. Ginsberg, MD,6 performed a randomized trial comparing lobectomies with limited resection for stage I lung cancer (tumor diameter ≤ 3 cm without lymph node metastases; LCSG 821). In this study, 276 patients were randomized to the trial arms; however, only 247 were analyzed due to protocol violations. The local recurrence rate was 3-fold higher in the limited resection group compared to the lobectomy group (5.4%/person/year vs. 1.9%), whereas there was no difference in distant recurrence rates.6 In addition, OS was longer in the lobectomy group with a one-sided p-value of 0.062.6 Limited pulmonary resection also failed to confer improved perioperative morbidity or mortality, or reduction in late postoperative complications.6 Therefore, the authors concluded that lobectomy was still the surgical procedure of choice for patients with peripheral T1N0 NSLC. However, this paper carried several flaws in that there was no significant difference if all randomized patients were analyzed and that pulmonary function tests were not systematically completed, as pointed out in accompanying commentaries. However, lobectomy has remained the standard of care based in large part on this paper because this was the only phase III randomized study at that time to address this issue.
Since then, limited resections for NSCLC are “officially” indicated only for very early-stage tumors or for patients with functional organ impairment who would not tolerate lobectomies. The systematic quantitative review analyzing 53 non-randomized studies and the aforementioned LCSG study showed that there was no difference in OS between lobectomies and sublobar resections in patients intentionally selected by oncologic features of the tumor, but that there was a significantly worse outcome in the patients undergoing sublobar resections because of their poor functional reserve.7
Postoperative Complications and Pulmonary Functions After Sublobar Resections
Although the LCSG study did not show improvement in pulmonary function in the sublobar group, Okada et al.8 showed that sublobar resection for tumors 2 cm or smaller resulted in similar survival outcomes, yet significantly better postoperative pulmonary function compared with patients who underwent lobectomies in their prospective non-randomized study. Postoperative acute exacerbation (AE) of interstitial lung disease (ILD) is one of the main causes of postoperative mortality following pulmonary resections for lung cancer. In a large retrospective analysis of 1,763 patients with ILD who underwent pulmonary resections, larger surgical procedures were significantly associated with greater chance of AE (odds ratio of lobectomy/segmentectomy compared to wedge resection was 3.83; p < 0.001).9
Technical Considerations
As mentioned earlier, it is important to secure an adequate margin of intact tissue in cancer surgery of any organ. With respect to lung cancer procedures, difficulties for securing such margins depend on the size as well as depth of the tumor from the surface of the lung. This is because the lung structure is understood as a collection of pyramid-shaped segments with their bottoms located toward the surface of the lung and with their apices toward the pulmonary hilum. Therefore, the deeper and the bigger a tumor is, the more difficult it will be to perform “adequate” sublobar resections, especially wedge resections. Indeed, in retrospective studies, it has been shown that tumors larger than 2 cm have a higher local recurrence rate.10
Role of Ground-Glass Appearance on CT for Selection of Patients for Sublobar Resections
However, it has often been observed that even a small tumor less than 2 cm may show aggressive tumor behavior. In 1995, Noguchi11 reported the subclassification of the adenocarcinoma of the lung less than 2 cm according to histologic findings. In Noguchi’s classification, type A, which featured replacement growth of alveolar-lining epithelial cells with a relatively thin stroma, and type B (type A with foci of structural collapse of alveoli) yielded a 100% 5-year survival rate.11 With the great improvement in the resolution of modern CT imaging, a tumor with this replacement growth manifests as a nodule with a pale radio-opaque shadow resembling frosted glass. These are called ground glass nodules (GGN). GGN can be inflammatory; however, if such shading persists for a long period of time over serial scans, it is very likely to be early lung adenocarcinoma without invasion.12,13
In several retrospective studies, it was proposed that the ratio of the diameter of a consolidated part (non–ground glass) to that of a total tumor (C/T ratio) was associated with patient prognosis (Fig. 2). The Japan Clinical Oncology Group (JCOG) 0201 study prospectively showed that when radiologic invasiveness was defined as tumor diameter less than 2 cm with C/T ratio less than either 25% or 50%, 99% and 96% of tumors, respectively, were pathologically non-invasive, defined as the absence of invasion into blood vessels, lymphatics, and pleura or lymph node metastases.14 As expected, patients with tumors with C/T less than 25% had excellent survival outcomes, with a 5-year recurrence-free survival rate of 97% when treated by lobectomy.15 Following this study, sublobar resections (mainly wedge resections) for these radiologically non-invasive lung cancers were prospectively examined in the JCOG0804/WJOG4507L study.16 The 5-year recurrence-free survival for these 314 patients was 99.7% (95% CI [97.7%, 100.0%]) with no local relapses recorded.16
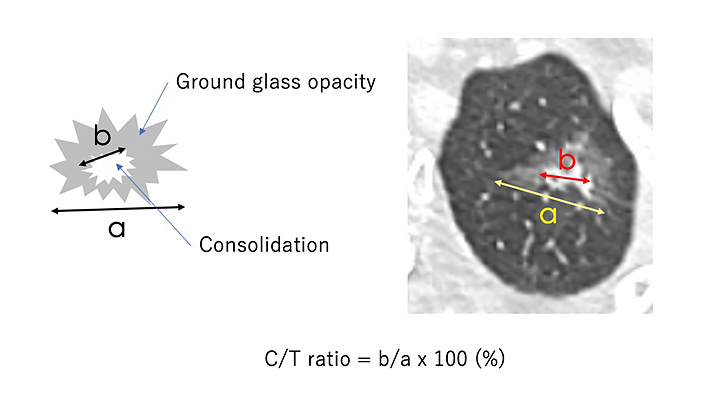
Abbreviations: C/T ratio, ratio of the diameter of a consolidated part (non-ground glass) to that of the total tumor.
To further expand the indication of sublobar resections for lung cancer, the JCOG, in a prospective, randomized trial, is now comparing lobectomies to segmentectomies for tumors 2 cm or smaller and a C/T ratio greater than 50% (JCOG0802/WJOG4607L). Patient accrual has completed, and data maturation are awaited. Similarly, CALGB 14503 is a randomized controlled trial comparing lobectomies to segmentectomies/wedge resection for peripheral NSCLC 2 cm or smaller in diameter with negative lymph node metastases.
Conclusion
Lobectomy remains the standard of care for pulmonary resection for lung cancer. However, sublobar resections are a reasonable option for selected patients with early lung cancer. According to the latest version of the National Comprehensive Cancer Network guideline, segmentectomies or wedge resections are considered appropriate for patients who would not tolerate lobectomies due to comorbidity or for those with peripheral nodules 2 cm or smaller with at least one of the following features: 1) pure adenocarcinoma in situ, 2) C/T ratio 50% or less, or 3) radiologically surveyed long tumor doubling time of 400 days or greater.17
About the Author: Dr. Mitsudomi is President of IASLC and a professor in the Department of Thoracic Surgery, Kindai University Faculty of Medicine, Japan.
References:
1. Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. JAMA. 1933;101(4):1371-1374.
2. Churchill ED, Sweet RH, Soutter L, et al. The surgical management of carcinoma of the lung; a study of the cases treated at the Massachusetts General Hospital from 1930 to 1950. J Thorac Surg. 1950;20(3):349-365.
3. Shimkin MB, Connelly RR, Marcus SC, et al. Pneumonectomy and lobectomy in bronchogenic carcinoma. A comparison of end results of the Overholt and Ochsner clinics. J Thorac Cardiovasc Surg. 1962;44:503-519.
4. Wilkins EW, Jr., Scannell JG, Craver JG. Four decades of experience with resections for bronchogenic carcinoma at the Massachusetts General Hospital. J Thorac Cardiovasc Surg. 1978;76(3):364-368.
4a. Dhanasopon AP, Salazar MC, Hoag JR, et al. Fate of Pneumonectomy Patients Variably Captured by Non-Small Cell Lung Cancer Staging System. Ann Thorac Surg 104:1829-1836, 2017
4b. Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 14:212-222, 2019
5. Jensik RJ, Faber LP, Milloy FJ, et al. Segmental resection for lung cancer. A fifteen-year experience. J Thorac Cardiovasc Surg. 1973;66(4):563-572.
6. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60(3):615-622; discussion 622-623.
7. Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer. 2015;89(2):121-132.
8. Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg. 2006;132(4):769-775.
9. Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2014;147(5):1604-1611 e3.
10. Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. 2005;129(1):87-93.
11. Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75(12):2844-2852.
12. Kushihashi T, Munechika H, Ri k, et al. Bronchioloalveolar Adenoma of the Lung: CT-Pathologic Correlation. Radiology. 1994;193(3):789-793.
13. Kuriyama K, Seto M, Kasugai T, et al. Ground-Glass Opacity on Thin-Section CT: Value in Differentiating Subtypes of Adenocarcinoma of the Lung. Am J Radiol. 1999;173(2):465-469.
14. Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol. 2011;6(4):751-756.
15. Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg. 2013;146(1):24-30.
16. Suzuki K, Watanabe S, Wakabayashi M, et al. A nonrandomized confirmatory phase III study of sublobar surgical resection for peripheral ground glass opacity dominant lung cancer defined with thoracic thin-section computed tomography (JCOG0804/WJOG4507L). J Clin Oncol. 2017;35:suppl. abstr. 8561.
17. NCCN Clinical Practice Guidelines in Oncology Non-Small Cell Lung Cancer Version 3, 2020. nccn.org. Accessed March 1, 2020.
Related Article: Lung Cancer Considered – Episode XXV – Mar 16, 2020 Dr. Marty Edelman Speaks With IASLC President Dr. Tetsuya Mitsudomi











