By Kara Nyberg, PhD
Posted: June 24, 2020
Pneumonitis is a well-known complication associated with immune checkpoint inhibitors (ICI). A collaborative effort between the U.S. Food and Drug Administration (FDA) and Synapse, a consultancy firm, retrospectively analyzed both clinical trial data and real-world data (RWD) taken from the Advocate Aurora Health System to elucidate risk factors for treatment-associated pneumonitis in patients treated with chemotherapy or ICI-based regimens.1
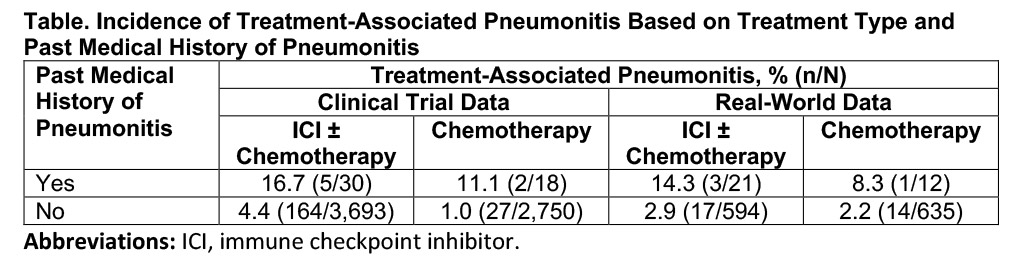
The findings revealed that two groups of patients with advanced NSCLC appear to develop treatment-associated pneumonitis at higher rates: (1) those with no history of pneumonitis treated with an ICI-based regimen as opposed to chemotherapy and (2) those with a prior history of noninfectious pneumonitis treated with either an ICI-based regimen or chemotherapy. Patients falling into the highest risk group had both a prior history of pneumonitis and received ICI-based therapy, where pneumonitis rates ranged from 14.3% to 16.7%.
In the real-world dataset, “most patients with a prior history of pneumonitis or treatment-associated pneumonitis received prior radiation therapy,” according to Qi Liu, PhD, MStat, of the FDA, who presented the current results. Given this observation, Dr. Liu indicated that future research efforts will focus on exploring past chest irradiation as a predictor of ICI-induced pneumonitis. In addition, the groups plan to leverage real-world data from other health system databases to expand the generalizability of the findings, as well as analyze the influence of treatment with tyrosine kinase inhibitors.
Of note, the increased incidence of ICI-induced pneumonitis among patients previously treated with thoracic radiation therapy has not been observed in ICI clinical trials such as KEYNOTE-001 and PACIFIC.2,3 “It appears there are data on both sides of the coin to suggest that prior radiation may be implicated in subsequent treatment-associated pneumonitis. This probably needs to be looked at further—most importantly in specific populations where there appears to be a higher risk, such as those with stage III lung cancer with oncogenic driver mutations,” said Jarushka Naidoo, MBBCh, MHS, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, who critiqued the study findings.
References:
1. Liu Q, Zhang C, Gong Y, et al. Pneumonitis incidence in patients with non-small cell lung cancer treated with immunotherapy or chemotherapy in clinical trials and real-world data. AACR Virtual Meeting; April 27-28, 2020. Abstract CT086.
2. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903.
3. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929.