By Kara Nyberg, PhD
Posted: June 24, 2020
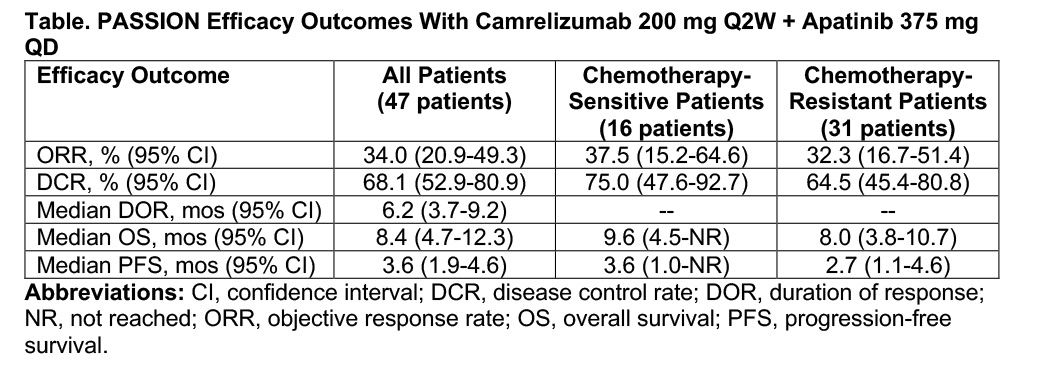
The phase II PASSION trial sought to explore the potential synergy between immunotherapy and antiangiogenic drug therapy previously observed in preclinical studies. As reported at AACR, the trial showed that combined second-line treatment with camrelizumab, a PD-1 inhibitor, and apatinib, a VEGFR2 inhibitor, demonstrated promising antitumor activity in patients with extensive-disease small cell lung cancer (ED-SCLC) who had previously received platinum-based chemotherapy.1 Moreover, the treatment duo appeared to be about equally effective in patients with chemotherapy-sensitive and chemotherapy-resistant disease (Table).
Three different dosing schedules of camrelizumab and apatinib were evaluated in PASSION. The majority of patients (47/59) received intravenous camrelizumab every 2 weeks in combination with oral apatinib once daily—the regimen that proved most efficacious but still tolerable in stage I testing.
The objective response rate with this regimen reached 34%. This was an encouraging finding considering that response rates with second-line topotecan and single-agent PD-1–directed therapy typically range from 9% to 23% among patients with ED-SCLC. The median duration of response (6.2 months) and median progression-free survival (3.6 months) were modest in this pretreated patient population.
Among all 59 patients treated with a camrelizumab-apatinib combination, the most common treatment-related adverse events of grade 3 or higher were hypertension (25.4%), hand-foot syndrome (13.6%), and decreased platelet count (13.6%).
Although the results support further evaluation of camrelizumab plus apatinib in the second-line SCLC setting, introducing angiogenesis inhibitors earlier in the treatment paradigm may make bigger improvements in this disease, especially since adding PD-1/PD-L1 targeted agents to frontline chemotherapy has now become standard practice.
Reference:
1. Wang J, Fan Y, Zhao J, et al. Camrelizumab plus apatinib in extensive-stage small-cell lung cancer (PASSION): A multicenter, two-stage, phase 2 trial. AACR Virtual Annual Meeting; April 27-28, 2020. Abstract CT083.