By Kara Nyberg, PhD
Posted: April 16, 2020
After decades of failed clinical trials and persistently dismal lung cancer survival outcomes, the 2010s breathed new life into the beleaguered field of lung cancer research. Treatment progress gained momentum and finally reached a tipping point in the mid-2010s, with the number of advances over the last 5 years outweighing all the advances in the 5 decades leading up to that point.
As the field continues to race forward into the 2020s, it seems fitting to reflect back on how much the treatment of lung cancer has evolved over the past decade.
Surgery
Surgical oncologists worldwide increasingly turned to video-assisted thoracic surgery (VATS) in the 2010s to manage early-stage lung cancer, with many centers now favoring this minimally invasive approach over open thoracotomy to reduce surgical morbidity.1 Multiple observational studies and meta-analyses pointed to fewer postoperative complications and better short- and long-term survival with VATS lobectomy compared with open lobectomy. However, data from a large randomized trial supporting the advantage of this approach were heretofore lacking—that is, until the British VIOLET study, the largest randomized trial ever to compare clinical outcomes following VATS versus open surgery in patients with early-stage disease.
At the end of 2019, the VIOLET investigators reported that patients who underwent VATS lobectomy experienced significantly fewer in-hospital complications compared with those who underwent open lobectomy (32.8% vs. 44.3%; p = 0.008), as well as a shorter length of stay (4 vs. 5 days; p = 0.008).2 Importantly, these benefits were attained without compromising early oncologic outcomes (i.e., R0 resection rates or lymph node upstaging) or increasing serious adverse events in the early postoperative period. Results for patient-reported pain, quality of life, and disease recurrence at 1 year are still awaited.
Given its success in treating inoperable lung cancer, ongoing research is now focused on whether SABR can be used in lieu of surgery in early-stage disease.
Some centers have explored other techniques to further decrease the invasiveness of surgery, including segmentectomy, single-port VATS, and robotic-assisted thoracic surgery, with promising signals of success.
Radiotherapy
For patients with early-stage NSCLC that is unsuitable for surgery, stereotactic ablative radiotherapy (SABR) offers an alternative. Both the American Society for Radiation Oncology3 and the European Society for Radiotherapy and Oncology4 established guidelines to standardize SABR delivery to patients with peripherally located, early-stage, node-negative NSCLC who are not candidates for surgery or who refuse to undergo surgery, cementing this modality as a standard of care over conventional external beam radiotherapy. Given its success in treating inoperable lung cancer, ongoing research is now focused on whether SABR can be used in lieu of surgery in early-stage disease. Two large randomized trials, the Joint Lung Cancer Trialist’s Coalition STABLE-MATES trial (NCT02468024) and the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial (NCT02984761), were launched during the last 5 years to compare SABR versus surgery in patients with operable stage I NSCLC.
Molecular Testing
Following the discovery of oncogenic drivers in lung cancer in the early 2000s and initial forays into developing tyrosine kinase inhibitors (TKIs) to target those mutations, the field has embraced molecular testing as a fundamental tool to guide and individualize treatment selection for patients. The first ringing endorsement of molecular testing came in 2013 when the College of American Pathologists, the IASLC, and the Association of Molecular Pathologists jointly released guidelines recommending EGFR and ALK analysis of either the primary tumor or a metastatic lesion for all patients with advanced-stage adenocarcinoma, regardless of clinical risk factors.5 The associations since expanded the guidelines in 2018 to include ROS1, BRAF, MET, RET, HER2, and KRAS, underscoring the wide breadth of targets and corresponding therapies now available for lung cancer treatment.6
A study conducted by the Lung Cancer Mutation Consortium in the United States elegantly illustrated the importance of screening for driver mutations as a standard component of the diagnostic workup for NSCLC.7 Of 733 patients with adenocarcinoma who underwent genotyping for 10 oncogenic drivers, 64% harbored a targetable driver mutation. Notably, patients with an oncogenic driver who received targeted therapy survived a median of 3.5 years, whereas patients with a driver mutation who did not receive targeted therapy survived a median of 2.4 years. Median OS for patients without a driver mutation was 2.1 years. A nationwide study conducted by the French Cooperative Thoracic Intergroup that included more than 17,600 patients with advanced NSCLC subsequently reported similar findings, bolstering the clinical benefit and prognostic utility of molecular profiling.8
Systemic Therapy
In 2010, only approximately 20% of patients with lung cancer were expected to live 5 years beyond their initial diagnosis, largely owing to the late onset of disease. Moreover, only two targeted therapies, gefitinib and erlotinib, were available to target just one driver mutation, EGFR. As of 2020, nearly 20 new agents—targeted therapies, checkpoint inhibitors, and anti-angiogenic agents—have transformed the treatment landscape (see the Drug Approvals timeline), and OS rates are beginning to creep upward as a result. In 2016, the 5-year OS rate had reached 23.5%, and it is expected to continue to its climb as an increasing number of patients hit the 5-year mark since the initial introduction of novel therapies.
Targeted therapy
The field of lung cancer research intensified the pace of targeted therapy development in the 2010s, rolling out agents directed against new oncogenic drivers and iteratively introducing more potent agents with higher barriers to genetic resistance.
Five first-line EGFR-targeted agents reflecting three generations of development have come to market since the identification of EGFR sensitizing mutations more than 15 years ago. The newest of these, osimertinib, has emerged as the frontrunner in the first-line setting in NSCLC in many regions of the world based on both efficacy and safety, displacing erlotinib, afatinib, gefitinib, and dacomitinib as a preferred standard of care. This is largely based on the results of the phase III FLAURA trial, which documented significant improvements in median progression-free survival (PFS; 18.9 vs. 10.2 months; p < 0.001) and median overall survival (38.6 vs. 31.8 months; p = 0.046) with osimertinib compared with erlotinib or gefitinib, in tandem with a milder toxicity profile, less frequent central nervous system progression, and improved post-progression outcomes.9,10
An array of treatment options likewise now exist for patients with ALK rearrangements. These include the first-generation ALK TKI crizotinib; the second-generation agents ceritinib, alectinib, and brigatinib; and the thirdgeneration agent lorlatinib. In the phase II study supporting lorlatinib approval for second- or later-line treatment of ALK-positive disease, objective response rates in patients previously treated with at least one ALK TKI reached 47%.12 Notably, among 81 patients with measurable brain lesions at baseline, lorlatinib yielded an objective intracranial response in 63%, with a median duration of response of 14.5 months.
Inhibitors of ROS1-rearranged NSCLC entered the scene in 2016 with the approval of crizotinib for ROS1-positive tumors. This has since been followed by the approval of entrectinib for ROS1-positive metastatic NSCLC based on an integrated analysis of three ongoing phase I and II trials (ALKA-372-001, STARTRK-1, and STARTRK-2). The analysis showed that entrectinib yielded an objective response in 77% of patients with ROS1 fusion–positive NSCLC and maintained that response for a median of 24.6 months.13
Other targeted therapies approved for metastatic NSCLC in just the last few years include dabrafenib/trametinib for patients with BRAF V600E mutation–positive disease, along with larotrectinib and entrectinib for patients with disease harboring the NTRK gene fusion who lack viable treatment options.
Immunotherapy
The introduction of immune checkpoint inhibitors in 2015 represents a major milestone in lung cancer treatment. At that time, nivolumab, pembrolizumab, and atezolizumab monotherapy each demonstrated the ability to prolong survival by approximately 2 to 3 months when pitted against the prior standard, docetaxel, in previously treated squamous and nonsquamous NSCLC in randomized trials.14-17 After becoming established for second- or later-line treatment, efforts quickly escalated to move immunotherapy into the first-line setting, along with routine testing for tumor PD-L1 expression, where the effects of checkpoint inhibitor therapy appeared to be more pronounced. Pembrolizumab was the first to break this new ground by demonstrating superior median PFS and OS compared with platinum-based chemotherapy in patients with high (≥ 50%) PD-L1 expression (10.3 vs. 6.0 months; p < 0.001).18 Since that time, frontline use of a checkpoint inhibitor, either alone for tumors with high PD-L1 expression or in combination with chemotherapy regardless of PD-L1 tumor expression, has now become a standard of care for patients with advanced NSCLC lacking a driver mutation, followed thereafter by continuation of immunotherapy for at least 2 years in an effort to maintain response.
The checkpoint inhibitor breakthroughs do not stop there. Durvalumab first established a new standard of care in unresectable stage III NSCLC based on evidence that use of the immunotherapy as consolidation following the completion of chemoradiotherapy significantly prolonged both OS (2-year OS: 66.3% vs 55.6%; p = 0.005) and PFS (median: 16.8 vs 5.6 months; p < 0.001) as compared with placebo.19 in 2018, checkpoint inhibitors made headway in extensive-stage SCLC, with atezolizumab being the first to significantly prolong median OS when combined with carboplatin/etoposide, as compared with carboplatin/etoposide alone (12.3 vs. 10.3 months; p = 0.007).20
Anti-angiogenic therapy
In 2014, ramucirumab became the second anti-angiogenic agent to enter the NSCLC treatment landscape after bevacizumab, which was originally approved for NSCLC in 2006. Authorization of the VEGFR-2 inhibitor was based on the phase III REVEL trial conducted in more than 1,200 patients with squamous and nonsquamous NSCLC whose disease progressed during or after a first-line platinum-based regimen. Patients who received docetaxel plus ramucirumab realized superior outcomes compared with patients who received docetaxel plus placebo, both for median OS (10.5 vs. 9.1 months; p = 0.023) and median PFS (4.5 vs. 3.0 months; p <0.0001).21
Supportive/Palliative Care
The importance of early palliative care for ambulatory patients with metastatic NSCLC first came to light in 2010. In a randomized trial that included 151 patients, the many benefits of combining early palliative care with standard oncologic care, compared with standard oncologic care alone, included better patient quality of life on the Functional Assessment of Cancer Therapy–Lung scale (98.0 vs. 91.5; p = 0.03), fewer depressive symptoms (16% vs. 38%; p = 0.01), and longer median OS (11.6 vs. 8.9 months; p = 0.02) despite a lower likelihood of aggressive end-of-life care (33% vs. 54%; p = 0.05).22 Following corroboration by subsequent studies, concurrent palliative care is now recommended as a standard component of disease management in tandem with active treatment for all patients with advanced lung cancer.23 ✦
References:
1. Cao C, Frick AE, Ilonen I, et al. European questionnaire on the clinical use of video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2018;27(3):379-383.
2. Lim E, Batchelor T, Dunning J, et al. In hospital clinical efficacy, safety and oncologic outcomes from VIOLET: A UK multi-centre RCT of VATS versus open lobectomy for lung cancer. In: Program and Abstracts of the 2019 World Conference on Lung Cancer; September 7-10, 2019; Barcelona, Spain. Abstract PL02.06.
3. Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(5):295-301.
4. Guckenberger M, Andratschke N, Dieckmann K, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol. 2017;124(1):11-17.
5. Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823-859.
6. Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol. 2018;13(3):323-358.
7. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998-2006.
8. Barlesi F, Mazieres J, Merlio JP, et al; Biomarkers France contributors. Routine molecular profi ling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387(10026):1415-1426.
9. Soria JC, Ohe Y, Vansteenkiste J, et al; FLAURA Investigators. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125.
10. Ramalingam SS, Vansteenkiste J, Planchard D, et al; FLAURA Investigators. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41- 50.
11. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
12. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654-1667.
13. Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-smallcell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):261-270.
14. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135.
15. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639.
16. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
17. Fehrenbacher L, Spira A, Ballinger M, et al; POPLAR Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846.
18. Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
19. Antonia SJ, Villegas A, Daniel D, et al; PACIFIC Investigators. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342-2350.
20. Horn L, Mansfield AS, Szczęsna A, et al; IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensivestage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229.
21. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673.
22. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
23. Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non–small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(30):3488-3515.
Lung Cancer Screening
Source: Am J Prev Med. Author manuscript; available in PMC 2020 Jan 1. Published in final edited form as: Am J Prev Med. 2019 Jan; 56(1): 66–73. Published online 2018 Nov 19. doi: 10.1016/j.amepre.2018.07.030
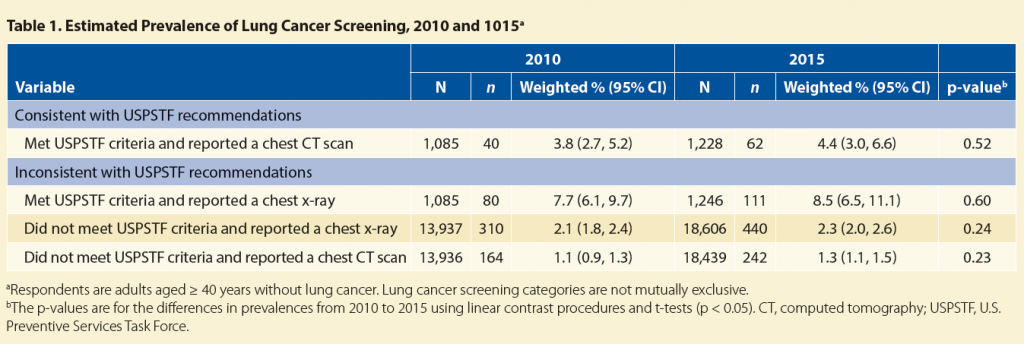
In 2015, the estimated prevalence of lung cancer screening with chest CT was about 4% among individuals meeting USPSTF smoking status and age criteria (55−80 years; Table 1).
Source: Am J Prev Med. Author manuscript; available in PMC 2020 Jan 1. Published in final edited form as: Am J Prev Med. 2019 Jan; 56(1):66–73. Published online 2018 Nov 19. doi: 10.1016/j.amepre.2018.07.030
In 2010, the National Lung Cancer Screening Trial (NLST) became the first study ever to document improvements in lung cancer mortality through use of a specific screening approach.1 Among 53,454 ever-smokers aged 55 to 74 years who had at least a 30 pack-year history and who quit no more than 15 years previously or who still smoked, the individuals randomly assigned to screening via low-dose chest CT demonstrated a 20% reduction in lung cancer mortality and a 6.7% reduction in overall mortality when compared with individuals randomly assigned to screening with a plain chest x-ray. Following the publication of these results, the US Preventive Services Task Force officially recommended yearly low-dose CT screening for patients aged 55 to 77 with at least a 30 pack-year history who had smoked within the last 15 years; however, community uptake of this approach has been slow and rates of low-dose CT screening remain low.2 ✦
References:
1. The National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
2. Okereke IC, Nishi S, Zhou J, Goodwin JS. Trends in lung cancer screening in the United States, 2016–2017. J Thorac Dis. 2019;11(3):873-881.











