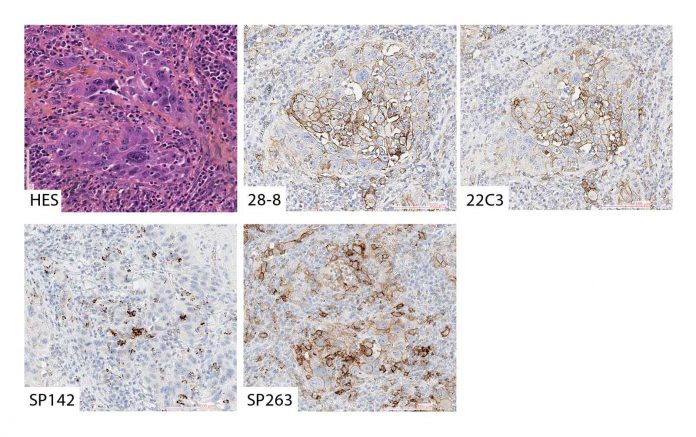
An example of solid and acinar type adenocarcinoma stained with Hematoxylin Eosin Saffron stain (A), the 28.8 assay on DAKO platform (B), the 22C3 assay on DAKO platform (C), the SP142 assay on Ventana platform (D) and the SP263 assay on Ventana platform (E). B, C and E shows membranous staining of PD-L1 on >50% of tumor cells (TPS >50%), while scattered immune cells but no tumor cells are positive for PD-L1 in D (TPS<1%). Original magnification x200 for all.
By Sylvie Lantuejoul, MD, PhD, and Mari Mino-Kenudson, MD
Posted: April 16, 2020
Immune checkpoint inhibitors (ICI), which target the PD-1/PD-L1 axis with the aim of restoring anti-tumor immunity, are now available in routine clinical practice for the treatment of patients with advanced or metastatic NSCLC and SCLC. Several biomarkers have been reported to predict clinical response, but to date, only PD-L1 expression assessed by immunohistochemistry (IHC) has been validated as a companion or complementary diagnostic to identify patients who are more likely to benefit from those therapies. Because many articles have been published since release of the IASLC Atlas of PD-L1 Immunohistochemistry Testing in Lung Cancer,1 the Immune Biomarker Working Group has proposed to the IASLC Pathology Committee to provide updates on the indications of ICIs for lung cancer in 2019 and a state of science review on PD-L1 IHC assays. The aim was to discuss important considerations on PD-L1 IHC, including issues affecting the quality of the testing at pre-analytical, analytical, and post-analytical steps.
Assays: Availability and Interchangeability
The first part of the review, written by thoracic oncologists, summarizes the available immunotherapy-based treatment strategies indicated in first-line and second- or later-line treatment of metastatic or locally advanced NSCLC and SCLC as single-agent immunotherapy or in combination with chemotherapy. Subsequently, four commercial PD-L1 IHC assays—22C3, 28-8, SP142, and SP263—are described, and the predictive role and prognostic value of PD-L1 expression evaluated in clinical trials are discussed. Of note, the four assays were codeveloped and evaluated along with a specific PD-1/PD-L1 agent (pembrolizumab, nivolumab, atezolizumab, and durvalumab, respectively) in clinical trials and have been approved as a companion or complementary diagnostic for the corresponding agent. The review addresses the important practical question about whether the combination of chemotherapy plus immunotherapy is better than immunotherapy alone for patients with the PD-L1 tumor proportion score (TPS) of 50% or greater and emphasizes the importance of PD-L1 testing in determining first-line treatment strategies between pembrolizumab monotherapy and the combination of immunotherapy and chemotherapy after the implementation of the combination therapy for patients with advanced NSCLC.
Given the impracticality of laboratories running multiple PD-L1 assays for the corresponding PD-1/PD-L1 agent, the question of comparability and interchangeability of these assays is also addressed and illustrated by a comparison of the different tests available on different platforms. In a nutshell, there have been at least 14 studies comparing the analytical (staining) performance of the four commercial PD-L1 IHC assays; essentially all studies that included the SP142 assay have reported its lower sensitivity in detecting PD-L1 expression in tumor cells and sometimes in immune cells as compared to all other assays (Fig.). Among studies that compared 22C3, 28-8, and SP263 assays, some noted good concordance between all three assays in detecting PD-L1 staining on tumor cells, while others reported the highest level of concordance between 22C3 and 28-8 assays with greater sensitivity of SP263 than those of 22C3 and 28-8 in detecting tumor cell PD-L1 expression. The possible difference in the sensitivity between SP263 and 22C3 may have clinical implications, if SP263 and 22C3 are used to select patients eligible for pembrolizumab and durvalumab therapy, respectively. In addition, because not all laboratories are equipped with the dedicated IHC platforms, many laboratories have set up in-house or laboratory-developed tests (LDTs), which are more affordable than the generally expensive clinical trial‒ validated commercial assays. The review discusses most studies comparing technical performance between LDTs and the commercial assays with clear evidence that the majority of LDTs are equivalent to the commercial assays from an analytical perspective; nevertheless, an adequate validation with an appropriate standard (a commercial assay) as a reference must be carried out before implementation of an LDT.
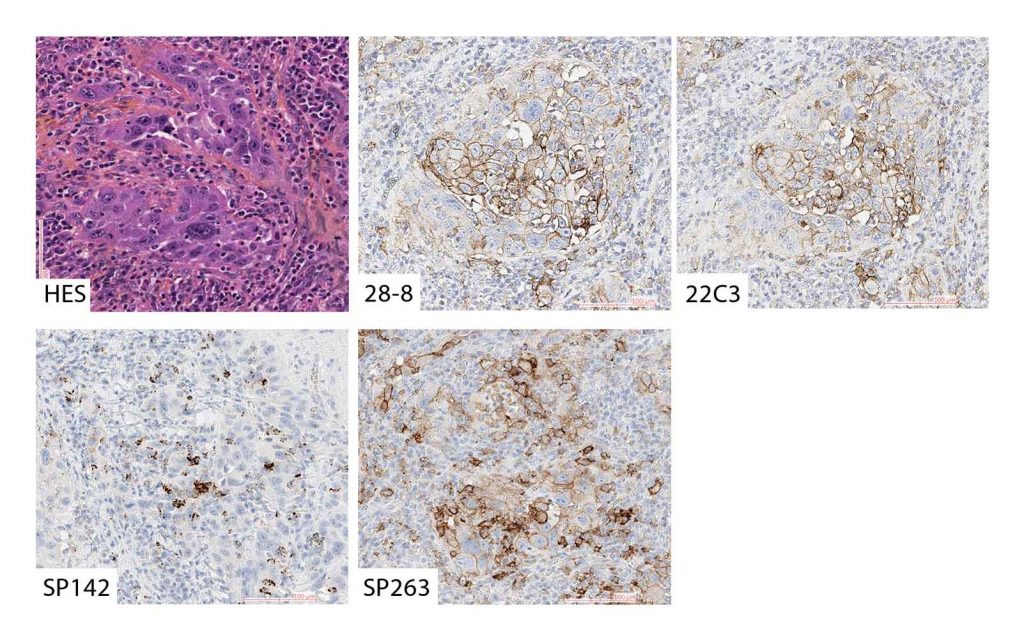
An example of solid and acinar type adenocarcinoma stained with Hematoxylin Eosin Saffron stain (A), the 28.8 assay on DAKO platform (B), the 22C3 assay on DAKO platform (C), the SP142 assay on Ventana platform (D) and the SP263 assay on Ventana platform (E). B, C and E shows membranous staining of PD-L1 on >50% of tumor cells (TPS >50%), while scattered immune cells but no tumor cells are positive for PD-L1 in D (TPS<1%). Original magnification x200 for all.
General Recommendations
The review also provides general considerations on pre-analytical steps of the IHC technique, including cold ischemia time, quantity and quality of fixative, fixation time, storage conditions, and age of archived unstained sections/blocks as well as sample types. Although the use of cytologic specimens for PD-L1 testing has not been validated in clinical trials, cytology samples may be the only specimen available for PD-L1 testing in many patients with advanced NSCLC. Consequently, multiple studies have looked at the performance of cell blocks and/or other cytology preparations for PD-L1 testing compared to surgical samples and reported high concordance in PD-L1 expression with a 50% cut-off between the two specimen types irrespective of assays used. Appropriate IHC protocols for cytology materials, however, should be first validated and submitted to quality-control measures. If the cytology laboratory offers more than one format (e.g., cyto-spins and cell blocks), the protocols must be developed accordingly with any pretreatment, including antigen-retrieval conditions for multiple fixatives and optimization of dilutions. Further, intratumoral and intertumoral heterogeneity of PD-L1 expression in association with the representativity of small samples such as cytology specimen and biopsies is discussed, and recent data from the literature on the effects of chemoradiotherapy on PD-L1 expression are introduced. The review briefly touches on possible inter- and intraobserver variabilities on PD-L1 assessments, given the semiquantitative nature of assay scoring. It assures the good agreement on PD-L1 tumor cell scoring using various assays and cut-offs but reveals the poor agreement on immune cell scoring in NSCLC specimens. Last but not least, the review emphasizes the importance of the pathology laboratory’s participation in quality assurance program(s), as well as the importance of the pathologist attending at least one of multiple online or formal hands-on training sessions to improve the quality and reproducibility of PD-L1 testing.
Although PD-L1 IHC may not be a perfect biomarker for immunotherapy, it has been implemented in the vast majority of pathology laboratories, and it is widely used by clinicians to identify patients eligible for immunotherapy. Thus, it is important to meet the standard of pre-analytical, analytical, and post-analytical requirements discussed in the review to make PD-L1 IHC as reliable as possible. Appropriate clinical care depends on this. ✦
About the Authors: Dr. Mino-Kenudson is a director of the Pulmonary Pathology Service, Department of Pathology at Massachusetts General Hospital and a professor of pathology at Harvard Medical School. Dr Lantuejoul is a consultant in the Department of Biopathology at the Centre Léon Bérard, Lyon and a professor of biopathology at the Grenoble Alpes University, France.
Reference:
1. Tsao MS, Kerr KM, Dacic S, et al. IASLC Atlas of PD-L1 Immunohistochemistry Testing in Lung Cancer. https://www.iaslc.org/Portals/0/iaslc_pd-l1_atlas_mar2018_lo-res.pdf?ver=2019-06-06-153849-143. Published 2017. Accessed February 15, 2020.