By Amy C. Moore, PhD, and Upal Basu Roy, PhD, MPH
Posted: April 16, 2020


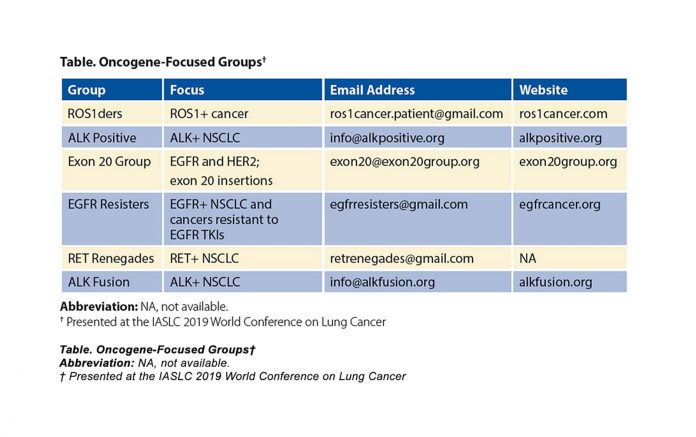
Genomic alterations drive more than 60% of adenocarcinoma cases of NSCLC.1 Approximately 25% of these cases will have an oncogenic driver (EGFR, ALK, ROS1, BRAF, or NTRK) that can be treated with approved targeted therapy drugs, and more (such as MET, RET, and exon 20 insertions) have clinical trial options.1 Patients and caregivers dealing with these cancers have organized globally into oncogene-focused groups (Table) and are building partnerships that seek to provide support, increase awareness and education, accelerate funding and research, and improve access to effective diagnosis and treatment (including access to clinical trials).2

The GO2 Foundation for Lung Cancer, its sister organization the Addario Lung Cancer Medical Institute (ALCMI), and the LUNGevity Foundation are effectively working in partnership with several of these oncogene groups, including the ROS1ders, the EGFR Resisters, and ALK Positive, to collaborate on research to address ongoing needs in the lung cancer community. Together with these groups as well as the Exon 20 Group, we recently presented our findings at the 2019 IASLC World Conference on Lung Cancer in Barcelona, where we shared the key characteristics required for building effective research collaborations:
1. Include patients from the start, in all aspects;
2. Address questions meaningful to patients;
3. Develop patient-centered measurements;
4. Accommodate patients’ clinical realities;
5. Leverage social media and patient groups;
6. Share progress with participants frequently; and
7. Make results rapidly and freely available.
Adherence to these seven key characteristics has enabled the patient groups to collaborate successfully with clinicians, researchers, advocacy organizations such as ours, and industry partners to generate ideas for next steps in research for their disease, forge new studies and clinical trials for a specific oncogenic driver, create new patient-derived models of oncogene-driven cancers to study acquired resistance, conduct studies to collect real-world data, and guide patients to clinical trials. Here we highlight ongoing efforts across the various groups to address unmet needs in the lung cancer community.
Developing Oncogene-Specific Cancer Models
Together with GO2 Foundation for Lung Cancer, ALCMI, Dr. Christine Lovly, Dr. Robert Doebele, and Champions Oncology, the ROS1ders are creating new ROS1 cell lines and patient-derived xenograft (PDX) mouse models from patient donations of excess fresh tumor tissue or pleural fluid. The ROS1 Cancer Model Project has already created four cell lines that have been distributed to researchers conducting research on ROS1 lung cancer.
Funding Oncogene-Specific Research
The ALK Positive group has partnered with LUNGevity Foundation to fund grants related to “improving the life expectancy and quality of life” for patients with ALK-positive NSCLC. Together with LUNGevity Foundation and leading thoracic oncology experts, the ALK Positive team selected the studies to be funded. These studies aim to understand how immunotherapy can be applied to ALK-positive lung cancer after progression on TKIs. The ALK Positive team is also funding another research project with GO2 Foundation focusing on using combination targeted therapies for ALK-positive tumors to delay or prevent drug resistance.
Treatment Sequencing
The Exon 20 Group has just initiated the Resistance Mutation Study and the Immune Cell Study, which aim to understand the resistance landscape for EGFR exon 20 insertions and HER2 exon 20 insertions. The project, which was funded by the Exon 20 group, will work with patients and families to focus on more effective sequencing of therapies.
Collecting Patient- Reported Data
The EGFR Resisters’ Project PRIORITY (Patient Reported Initiative On Resistance, Incidence, Treatment studY) is a survey-based project in collaboration with LUNGevity Foundation. It aims to understand the treatment experience of patients with EGFR-positive lung cancer, specifically, to identify areas for improvement in diagnosis and treatment and give voice to patient concerns regarding risk factors, symptoms, and side effects of treatments. Preliminary results of the study have already been presented at the 2019 IASLC World Conference on Lung Cancer and the 2019 IASLC North America Conference on Lung Cancer. Additionally, the ROS1ders have worked with GO2 Foundation to look at key characteristics of patients with ROS1-positive NSCLC using a patient-designed, global, pan-cancer data repository.3
As scientists working within lung cancer advocacy organizations, we know firsthand the progress that has been made in recent years in the treatment of this disease. Indeed, because of these advances, scientists recently reported the largest single-year drop in cancer mortality, driven largely by a decline in lung cancer deaths.4 Yet, we have been unable to signifi cantly move the needle on overall survival, and lung cancer research remains grossly underfunded. We must explore new paradigms of partnership and collaborative research if we are to drive innovation that truly affects patients with lung cancer. Together, we are committed to nurturing effective collaborations with these oncogene-focused patient groups to deliver meaningful outcomes that improve, extend, and save lives. ✦
About the Authors: Dr. Moore is the director of Science and Research, GO2 Foundation for Lung Cancer. Dr. Basu Roy is vice president of Research, LUNGevity Foundation.
References:
1. Pakkala S, Ramalingam SS. Personalized therapy for lung cancer: striking a moving target. JCI Insight. 2018;3(15):e120858.
2. Hennink M, Vandeweyer G, Freeman-Daily J; ROS1ders. The roles of patient groups in fostering cancer research. Nat Rev Clin Oncol. 2020;17(2):65-66.
3. Parikh DA, Walia G, Freeman-Daily J, et al. Characteristics of patients with ROS1+ cancers: results from the fi st patient-designed, global, pan-cancer ROS1 data repository. J Oncol Pract. Dec 27 2019. [Epub ahead of print].
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.