Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731–747.
By Alexander Drilon, MD
Posted: February 12, 2020
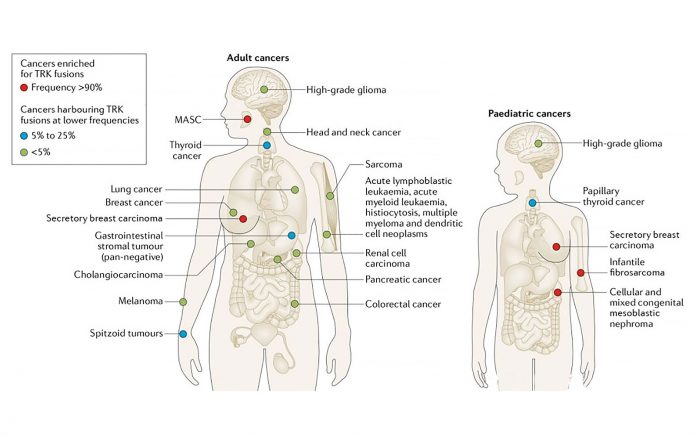
The receptor tyrosine kinases TRKA/B/C are encoded by the genes NTRK1/2/3. TRK fusions are oncogenic drivers of various adult and pediatric cancers. These fusions are found at high frequencies (> 90% in select series) in rare cancers such as secretory carcinoma and at lower frequencies in more common cancers.1
In NSCLCs, the estimated frequency of TRK fusions is 0.23%.2 TRK fusions are typically mutually exclusive with other drivers. These are found at a younger median age (48 years, range 25 to 86 years), seen in patients with minimal to no prior smoking history (median packyear history 0, range 0 to 58), and identified largely in adenocarcinomas. Similar to other drivers, however, all patients with NSCLC should be screened for TRK fusions regardless of these features given that not all patients fit this profile.
DNA-based next-generation sequencing (NGS) is an ideal first test to use; it is important to select an assay that reliably detects these alterations. It should be recognized that, due to technical reasons, DNA-based NGS can miss some TRK fusions. RNA-based sequencing is a complementary test that can detect these false-negative cases.3 In environments in which NGS is not available, immunohistochemistry (with a pan-TRK antibody) can be used to screen for TRK fusions, as many of these tumors will overexpress the oncoprotein.4 Other assays such as FISH, RT-PCR, and plasma ctDNA testing can also detect TRK fusions, but these assays have their own limitations.
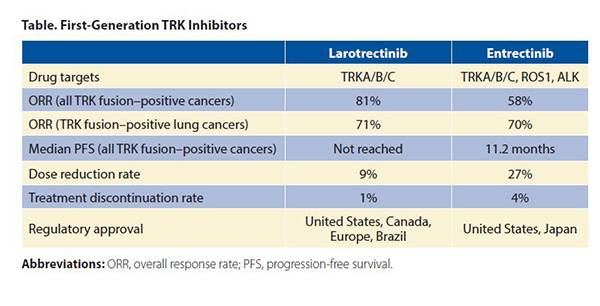
The first-generation TRK inhibitors (larotrectinib and entrectinib) are approved by several regulatory agencies for the treatment of TRK fusion–positive cancers in adult and pediatric patients. Larotrectinib is a selective TRK inhibitor, whereas entrectinib inhibits ROS1 and ALK in addition to TRK. The objective response rates (ORR) in all TRK fusion–positive cancers were 81%5 and 58%,6 respectively (Table). The median progression-free survival (PFS) was not reached for larotrectinib and was 11.2 months for entrectinib. Response rates in TRK fusion–positive NSCLCs were comparable (larotrectinib: ORR 71% [n = 5/7],7 entrectinib: ORR 70% [n = 7/10]8). Both drugs achieved intracranial responses in patients with central nervous system metastases (entrectinib: intracranial ORR 67% [n = 4/6] in NSCLC).8,9
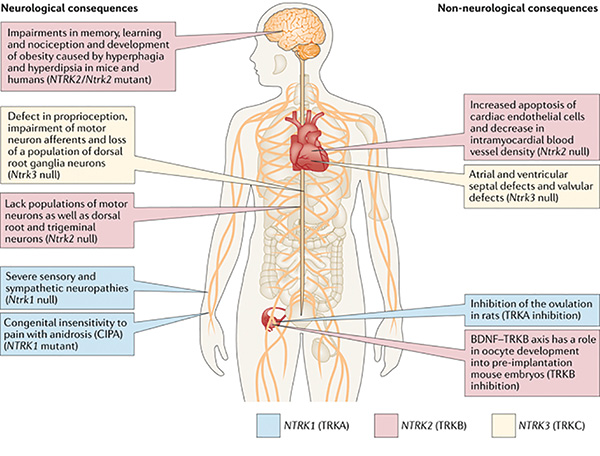
Larotrectinib and entrectinib have favorable safety profiles. Most adverse events were grade 1 or 2. Rates of dose reduction (larotrectinib: 9%, entrectinib: 27%) and treatment discontinuation (larotrectinib: 1%, entrectinib: 4%) were low compared to other targeted therapies.5,6 On-target adverse events mediated by TRK inhibition occasionally occur. These include weight gain, paresthesias, and dizziness/ataxia. In addition, pain flares can be observed in patients who temporarily or permanently discontinue TRK inhibitor therapy. These side-effects are predicted by the role that the TRK pathway plays in nervous system development and maintenance.1
Resistance to TRK inhibition can eventually occur. A proportion of cancers will acquire on-target resistance in the form of NTRK kinase domain mutations. These result in substitutions involving the solvent front, gatekeeper, and xDFG residues.1 Next-generation TRK inhibitors such as selitrectinib10 and repotrectinib11 have been developed to address several of these mutations. Both drugs are being evaluated in ongoing clinical trials (NCT03215511, NCT03093116), and clinical proof-of-concept cases (TRK fusion–positive cancers responding after progression on a prior TRK inhibitor) have been reported for both agents. This highlights the utility of a sequential paradigm of TRK inhibitor use in the appropriate context. Off-target resistance has also been described and can be mediated by activation of MET or the RAS-RAFMEK pathway.12
In summary, TRK fusions are clinically actionable drivers that are found in NSCLCs. Screening for TRK fusions should be performed in the clinic. Two first-generation drugs (larotrectinib and entrectinib) are currently approved for the treatment of TRK fusion–positive cancers in adult and pediatric patients. These drugs are well tolerated, although occasional and unique on-target side effects are observed. Next-generation TRK inhibitors that address on-target resistance are already in clinical trials. ✦
About the Author: Dr. Drilon is a medical oncologist, research director of Early Drug Development Service, and an associate attending physician of the Thoracic Oncology Service at Memorial Sloan Kettering Cancer Center.
References:
1. E Cocco, M Scaltriti, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731-747.
2. Farago AF, Taylor MS, Doebele RC, et al. Clinicopathologic Features of Non-Small-Cell Lung Cancer Harboring an NTRK Gene Fusion. JCO Precis Oncol. 2018 Jul 23. [Epub ahead of print].
3. Benayed R, Offin M, Mullaney K, et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin Cancer Res. 2019;25(15):4712-4722.
4. Hechtman JF, Benayed R, Hyman DM, et al. Pan-Trk Immunohistochemistry Is an Efficient and Reliable Screen for the Detection of NTRK Fusions. Am J Surg Pathol. 2017;41(11):1547-1551.
5. Tan DSW, Lassen UN, Albert CM, et al. Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach. Ann Oncol. 2018;29:viii133-viii148.
6. Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive tumours: Pooled analysis of STARTRK-2, STARTRK-1, and ALKA-372- 001. Ann Oncol. 2018;29:ix173-ix178.
7. Drilon A, Kummar S, Moreno V, et al. Activity of larotrectinib in TRK fusion lung cancer Ann Oncol. 2019;30(suppl_2).
8. Paz-Ares L, Doebele R, Farago AF, et al. Entrectinib in NTRK Fusion-Positive Non-Small Cell Lung Cancer (NSCLC): Integrated Analysis of Patients Enrolled in STARTRK-2, STARTRK-1 and ALKA-372-001. Ann Oncol. 2019;30 (suppl_2):ii38-ii68.
9. Drilon AE, DuBois SG, Farago, AF, et al. Activity of Larotrectinib in TRK Fusion Cancer Patients with Brain Metastases or Primary Central Nervous System Tumors. J Clin Oncol. 2019;37(15_suppl):2006.
10. Drilon A, Nagasubramanian R, Blake JF, et al. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017;7(9):963-972.
11. Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ ALK Inhibitor That Potently Inhibits ROS1/TRK/ ALK Solvent- Front Mutations. Cancer Discov. 2018;8(10):1227-1236.
12. Cocco E, Schram AM, Kulick A, et al. Resistance to TRK inhibition mediated by convergent MAP kinase pathway activation. Nat Med. 2019 Aug 12. [Epub ahead of print].