This article is the second in a two-part series on ongoing challenges using immunotherapy in special populations. The first part of this series focused on patients with poor performance status.
By Valérie Gounant, MD, and Elisabeth Quoix, MD, PhD
Posted: February 12, 2020


The advent of targeted therapies and immune checkpoint inhibitors (ICIs) was a major turning point in the management of advanced NSCLC. As mentioned in Part I of this series, the U.S. Food and Drug Administration (FDA) approvals of atezolizumab, nivolumab, and pembrolizumab were major breakthroughs in patient care for most, but use of these therapies in special populations remains challenging. The approvals for all three agents were based on the results of phase III clinical trials, in which very old patients were seldom included despite there being no upper age limit for eligibility.
Elderly patients are frequently included in special populations, although patients 70 years of age and older constitute 50% of all the patients with metastatic NSCLC, and those aged 75 years and older represent 30%.
Some theoretical arguments exist against the use of ICIs in elderly patients, especially the concept of immunosenescence.1 Immunosenescence may be one of the main explanations for the increased incidence of cancer with age and may affect the efficacy and toxicity of anticancer treatments. There is a highly dynamic remodeling of all of the immune functions as we age, with a decrease in the size of the thymus, and in the capacity of the bone marrow, lymph nodes, and spleen. Chronic antigenic stimulation during life is responsible for the transformation of naive T lymphocytes into memory cells; thus, there is a reduction of the antigenic diversity of immune cells with age, and the immune system of elderly people is less able to neutralize new antigens. Proliferation, but not the function, of T cells also decreases with age. Costimulatory molecules (CD28 and CD27) on T-lymphocytes are also reduced. B-lymphocytes are also modified with age, resulting in less production of antibodies with high affinity and specificity for antigens. On the other hand, secretion of auto-antibodies and pro-inflammatory cytokines is increased, leading to a state of low-grade chronic inflammation called “inflammaging.” Besides these modifications of adaptive immunity, immunosenescence also has an effect on innate immune response. Antigen-presenting cells have reduced ability to process antigens and have less expression of Toll-like receptors.
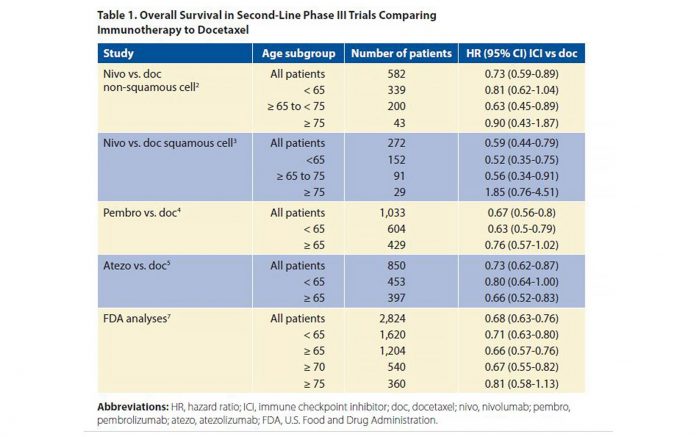
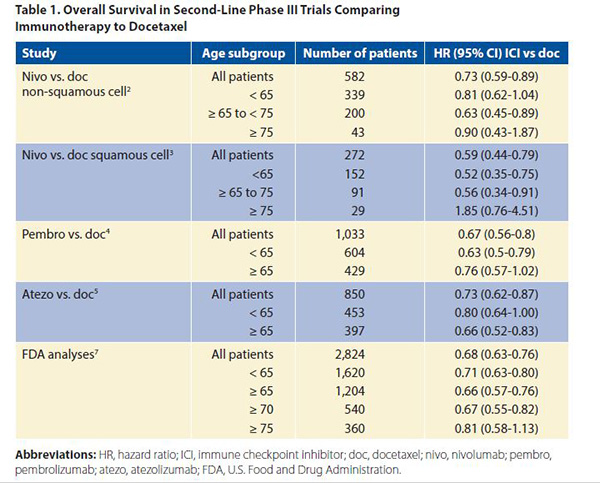
Each of the second-line phase III trials comparing the ICIs (nivolumab,2,3 pembrolizumab,4 atezolizumab,5 and avelumab)6 to docetaxel had no upper limit of age for inclusion, but were restricted to PS 0 or 1 patients. In these trials, which all favored immunotherapy over chemotherapy with respect to OS (with the exception of the JAVELIN trial testing avelumab), there was no dilution of the survival benefit when the cutoff was set at age 65 years. However, with age 75 as the cutoff, there was no significant OS benefit with immunotherapy in any of these trials (Table 1). Of note, the percentage of patients 75 and older was only 7%-10% of all patients enrolled; thus, definite conclusions cannot be drawn regarding very old patients given their under-representation on these trials and wide confidence intervals. The FDA created a pooled analysis of the survival of the 2,824 patients included in four trials comparing ICIs to docetaxel.7 These trials included three of the trials already discussed2,4,5 as well as the POPLAR phase II randomized trial comparing atezolizumab to docetaxel.8 They found that the treatment effects were similar across most age groups using both unadjusted and adjusted HRs. In particular, the upper bound of the 95% confidence intervals fell below 1 for patients aged 65 and older as well as for those aged 70 and older, but this was not the case for patients aged 75 and older (Table 1). Median survival time ranged between 14.1 months and 14.7 months for those receiving PD-1‒ and PD-L1‒ blocking antibodies versus 8.8 months and 9.5 months in the docetaxel “control” groups across the various age categories. Regarding adverse events in the anti‒PD-1/‒PD-L1 arms, the incidence of grade 3 to 4 adverse events appeared lower in patients aged 75 and older at 23%, compared to 49% of those aged 65 and older, and 47% of those younger than 65 years. Grade 5 toxicities were observed in 5%, 7%, and 4% of the patients, respectively. Similarly, there was no significant difference regarding adverse events of special interest (colitis, hepatitis, pneumonia, and hypothyroidism/elevated TSH) across each age groups. Thus, no signal of heightened toxicity was observed in patients aged 75 and older, although one must take into account that the very oldest enrollees on these studies were not numerous and probably highly selected, as they were obviously underrepresented based on demographic data. Regarding first-line therapy with ICIs, either given as a monotherapy in patients with a PD-L1 tumor proportion score of > 50%,9 or in association with chemotherapy,10 or chemotherapy and bevacizumab,11 the definitive phase II and III trials featured no upper age limit with respect to enrollment. In KEYNOTE 024, which compared pembrolizumab to standard chemotherapy, and in KEYNOTE 189, which compared pembrolizumab in combination with chemotherapy to chemotherapy alone, each of which showed a survival benefit overall, age had little bearing on outcome. A significant survival benefit was observed in both trials in favor of the pembrolizumab arm. With a cutoff of age 65, there was no difference in the treatment effect for the pembrolizumab arm compared to chemotherapy or for the pembrolizumab plus chemotherapy arm compared to chemotherapy alone. Regarding the trial comparing carboplatin plus paclitaxel plus bevacizumab to the same combination plus atezolizumab, a significant PFS and OS benefit was observed regardless of PD-L1 expression. Of the total patients included in this trial, 9.75% were aged 75 and older, but there was no analysis of the results according to age. A meta-analysis of all randomized trials comparing chemotherapy to immunotherapy or chemotherapy plus immunotherapy to chemotherapy alone was recently published12; it confirmed a PFS and survival gain in favor of immunotherapy. Using an age cutoff of 65 years, there was no significant difference in the magnitude of benefit between the two age categories, as expected.
A French Cooperative Thoracic Intergroup clinical trial for patients with metastatic NSCLC between the ages of 70 and 90 is currently recruiting. The trial compares chemotherapy with monthly carboplatin and weekly paclitaxel to the combination of this chemotherapy with atezolizumab (NCT03977194), and it will hopefully provide a definitive, prospective answer regarding the feasibility and utility of combined immunotherapy and chemotherapy in elderly patients. ✦
About the Authors: Dr. Gounant is with the Thoracic Oncology Department, Hôpital Bichat, APHP, France. Prof. Quoix is with the Department of Pneumology, University Hospital of Strasbourg, France.
References:
1. Ferrara R, Mezquita L, Auclin E, Chaput N, Besse B. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: Does age really matter? Cancer Treat Rev. 2017;60:60-68.
2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627-1639.
3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135.
4. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
5. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265.
6. Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468-1479.
7. Marur S, Singh H, Mishra-Kalyani P, et al. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin Oncol. 2018;45(4):220-225.
8. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846.
9. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1- Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823-1833.
10. Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078-2092.
11. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
12. Liu T, Ding S, Dang J, Wang H, Chen J Li G. First-line immune checkpoint inhibitors for advanced non-small cell lung cancer with wildtype epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK): a systematic review and network meta-analysis. J Thorac Dis. 2019;11(7):2899-2912.