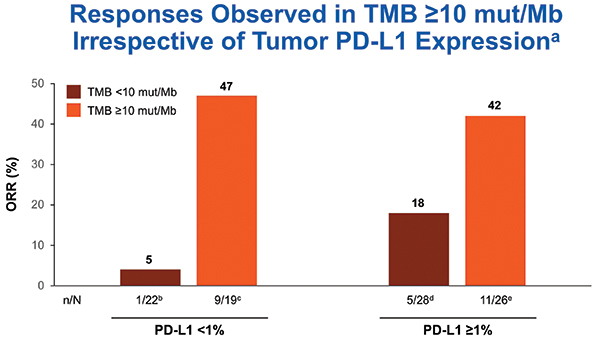
aIrrespective of TMB status; bORR was 33% in patients with non-quantifiable tumor PD-L1 expression (n = 36); cCR = 3%; dCR = 3%
By Neal Ready, MD, PhD
Posted: February 12, 2020

Programmed death receptor (PD-1) checkpoint monoclonal antibody therapy has proven a major advancement in the management of advanced NSCLC, with prolonged duration of response and significant 5-year survival rates not seen with combination platinum-based chemotherapy.1-6 CheckMate 012, CheckMate 568, and CheckMate 227 are a series of phase I, phase II, and phase III trials aimed at broadening the options for immunotherapy in NSCLC, including studying the immunotherapy combination of the PD-1 inhibitor nivolumab plus the CTLA-4 inhibitor ipilimumab.7-9 The studies investigated toxicity, efficacy, biomarkers, and the optimal phase II/III regimen.
CheckMate 012 was a multi-institutional phase I trial including treatment arms combining nivolumab plus ipilimumab every 3 weeks, 6 weeks, or 12 weeks. Nivolumab (3 mg/kg every 2 weeks) plus ipilimumab (1 mg/kg every 6 weeks) was chosen for phase II/III clinical trial development based on tolerability, promising efficacy, and evidence from other tumor types in which greater ipilimumab exposure was associated with improved activity.
CheckMate 568 was a phase II study with 288 patients with untreated recurrent or metastatic NSCLC. Patients were unselected for histology or programmed death receptor ligand-1 (PD-L1) status, and the study excluded known EGFR or ALK sensitizing alterations. Therapy consisted of nivolumab (3 mg/kg every 2 weeks) and ipilimumab (1 mg/kg every 6 weeks) for up to 2 years. The primary endpoint was overall response rate (ORR) by blinded independent central review (BICR) per RECIST v1.1 with required confirmation at 4 weeks or later, assessed in patients with > 1% or greater or < 1% tumor PD-L1 expression. Secondary endpoints included PFS, OS, toxicity, and efficacy by PD-L1 expression and total mutational burden (TMB).
TMB and PD-L1 Correlation, or Lack Thereof
The ORR of 30% by BICR for all treated patients confirmed the apparent increased efficacy reported in CheckMate 012 for nivolumab plus ipilimumab compared to nivolumab alone.7 The grade 3 or higher toxicity rate of 30% was also consistent with published data for nivolumab plus ipilimumab every 6 or 12 weeks.7
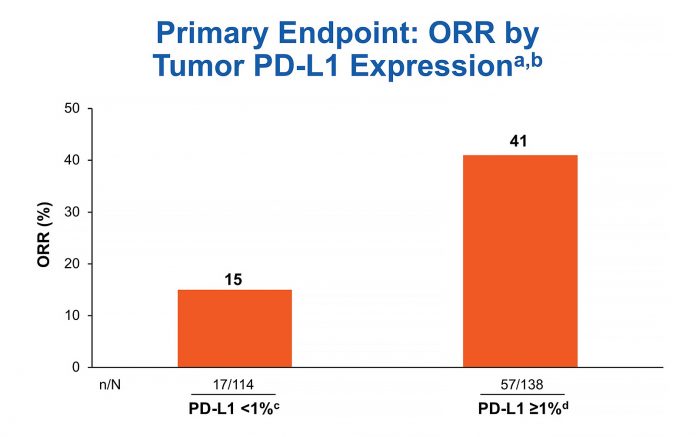
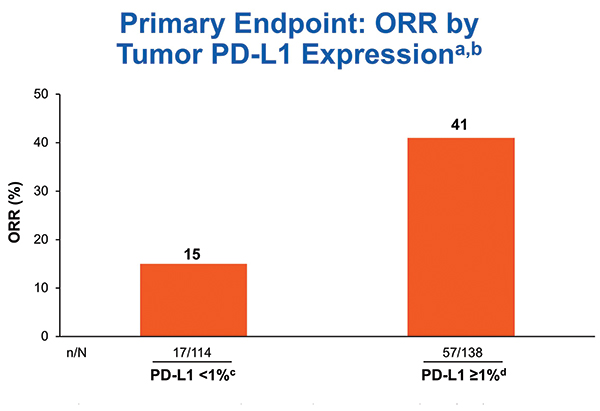
Biomarker testing was prioritized for PD-L1 first and TMB second. Out of 288 treated patients, 252 (87.5%) were successfully tested for PD-L1. The ORR for the 138 tumors with PD-L1 expression > 1% was 41% in CheckMate 568 (Fig. 1), comparing favorably to the ORR rate of 28% for the 32 tumors with PD-L1 expression > 1% receiving nivolumab in CheckMate 012 and 27% for the 637 tumors with PD-L1 expression of 1% or greater receiving pembrolizumab in KEYNOTE-042.8, 10, 11
aIrrespective of TMB status; bORR was 33% in patients with non-quantifiable tumor PD-L1 expression (n = 36); cCR = 3%; dCR = 3%
TMB was tested only after tumors were tested for PD-L1. TMB testing required 10 unstained slides and was conducted using the U.S. Food and Drug Administration–approved companion diagnostic next generation sequencing– based FoundationOne CDx assay. At least 10 slides were available for 121 cases, and 98 of those (82%) were successfully tested for TMB. Nonparametric receiver operating characteristic curve estimates were used to evaluate the performance of TMB as a classifier of ORR per BICR. A regression analysis demonstrated no association between TMB and PD-L1 expression, consistent with results from the CheckMate 026 and 227 studies.12, 9 In the TMB-evaluable population, ORR increased in subgroups of patients with higher TMB, plateauing at > 10 mut/Mb or greater. ORR was 44% versus 12% in patients with TMB >10 mut/Mb versus TMB < 10 mut/Mb. No additional ORR benefit was observed in patients with TMB > 15 mut/Mb (Fig. 2). PFS was superior for tumors with TMB > 10 mut/Mb compared to tumors with < 10 mut/Mb. Therefore, TMB > 10 mut/Mb was chosen as the cutoff for TMB bio-marker analysis. The association of efficacy with TMB did not depend on tumor PD-L1 expression (Fig. 3).
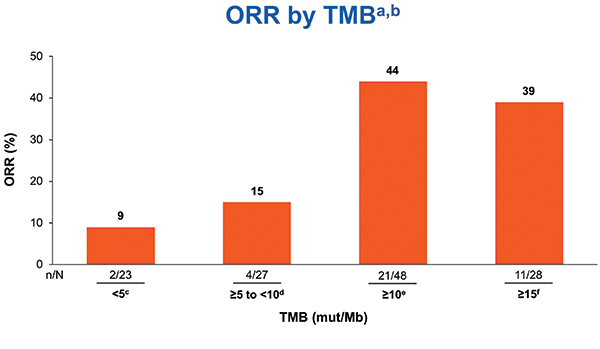
aIrrespective of PD-L1 expression; b12%ORR for < 10 mut/Mb and 50% ORR for ≥ 10 to < 15 mut/Mb;
cCR = 0; dCR = 4%; eCR = 8%; fCR = 7%
aORR for all treated patients: 41% in PD-L1 ≥ 1% subgroup (n = 138) and 15% in PD-L1 < 1% subgroup (n = 114); bCR = 0; cCR = 16%; dCR = 4%; eCR = 4%
Implications for Future Study
Approximately 40% of tested tumors had TMB > 10 mut/Mb, and the ORR was 44% for TMB-high tumors. The ORR was 47% in TMB > 10 mu/Mb tumors with PD-L1 expression < 1%. Thus, CheckMate 568 data suggested impressive activity for nivolumab plus ipilimumab in PD-L1– negative NSCLC with TMB > 10 mu/Mb. Approximately 40% of advanced NSCLC is PD-L1 negative, and approximately 40% of those tumors would be expected to have TMB > 10 mut/Mb, resulting in 10% to 20% of NSCLCs being both PD-L1 negative and TMB high by the Foundation Medicine assay. Patients with tumors that had PD-L1 expression < 1% and TMB < 10 mut/Mb had ORR of only 5%, suggesting that using the combination PD-L1 and TMB testing could identify a group of patients with a low likelihood of benefiting from combination PD-1 and CTLA-4 checkpoint blockade.
The TMB 10 mut/Mb cutoff identified in CheckMate 568 informed the statistical plan for the co-primary endpoint of PFS in patients with TMB > 10 mut/Mb in CheckMate 227. Patients with TMB > 10 mut/Mb had significantly prolonged PFS with first-line nivolumab plus ipilimumab versus chemotherapy; this validated the TMB cutoff identified in CheckMate 568.9 No significant difference in PFS between the two treatments was observed in patients with TMB < 10 mut/Mb.
CheckMate 568 demonstrates that the combination of nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks was tolerable with superior efficacy compared to historical outcomes for nivolumab monotherapy in untreated advanced stage NSCLC. TMB > 10 mut/ Mb was a cutpoint for ORR, and there was no evidence of increased efficacy for tumors with TMB > 15 mut/Mb. PD-L1 and TMB were independent predictive biomarkers. TMB > 10 mut/Mb was validated as a predictive biomarker when prospectively applied to CheckMate 227, and it showed improved PFS for nivolumab plus ipilimumab compared to standard chemotherapy. Nivolumab plus low dose ipilimumab produced durable responses by BICR in more than 40% of PD-L1–positive or TMB > 10 mut/Mb tumors. However, TMB has not consistently been predictive of improved overall survival with combination therapy. The part 1 results of Checkmate 227 presented at the 2019 European Society for Medical Oncology Annual Meeting in Barcelona, Spain showed an overall survival benefit for the nivolumab and low dose ipilimumab immunotherapy combination compared to chemotherapy.13 Consistent with the results for CheckMate 568, the ORR with nivolumab plus ipilimumab in CheckMate 227 was higher for PD-L1 > 1% tumors compared to PD-L1 < 1% tumors. However, the relationship between the PD-L1 biomarker and efficacy with the nivolumab and low dose ipilimumab combination is complex, since in CheckMate 227 there was a similar survival advantage for nivolumab and low dose ipilimumab compared to standard chemotherapy in PD-L1 positive and PD-L1 negative tumors. ✦
About the Author: Dr. Ready is a professor of medicine at Duke University School of Medicine.
References:
1. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627-1639.
2. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123-135.
3. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
4. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomized controlled trial. Lancet. 2017;389(10066):255-265.
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823-1833.
6. Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol. 2018;36(17):1675-1684.
7. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31-41
8. Ready N, Hellmann MD, Awad MM, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol. 2019;37(12):992-1000.
9. Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378(22):2093-2104.
10. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34(25):2980-2987.
11. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830.
12. Carbone DP, Reck M, Paz-Ares L, et al. CheckMate 026 Investigators. First-Line Nivolumab in Stage IV or Recurrent Non- Small-Cell Lung Cancer. N Engl J Med. 2017;376(25):2415-2426.
13. Peters S, Ramalingam S, Paz-Ares L, et al. Nivolumab + Low-Dose Ipilimumab Versus Platinum-Doublet Chemotherapy as First-Line Treatment for Advanced Non-Small Cell Lung Cancer: CheckMate 227 Part 1 Final Analysis. European Society for Medical Oncology (ESMO) 2019 Congress in Barcelona, Spain (Presentation #LBA4).










