By Robert C. Doebele, MD, PhD
Posted: December 11, 2019

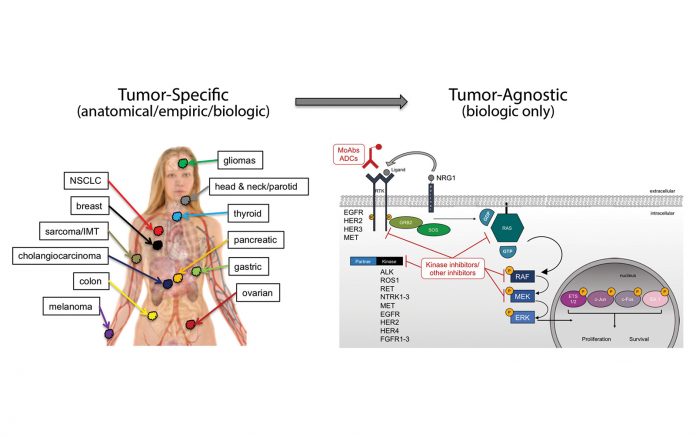
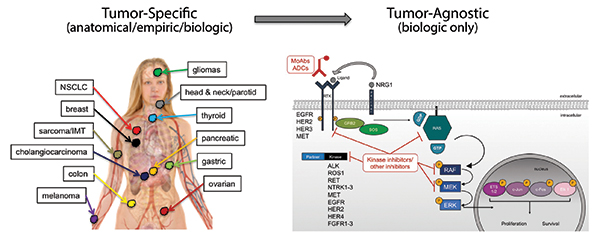
Early in the drug development of targeted therapies, specific oncogene mutations were often associated with a single disease: HER2 gene amplification with breast cancer, BCR-ABL fusions with chronic myelogenous leukemia, EGFR mutations with lung cancer, and BRAF mutations with melanoma. Thus, drug development and approval proceeded in both a mutation- and tumor-specific context for each of these indications. Through the efforts of The Cancer Genome Atlas, the Genomics Evidence Neoplasia Information Exchange, and other largescale pan-tumor sequencing efforts, as well as through the implementation of multiplexed next-generation sequencing panels in the clinic, it has become clear that specific oncogene mutations often occur in more than one tumor type. This revelation has opened the door for novel, tumor-agnostic, drug-development strategies.
BRAF
BRAF inhibitors alone or in combination demonstrate significant clinical benefit in patients with melanoma whose tumors harbor BRAF V600E. However, an early and prominent setback for the concept of tumor- (or tissue-) agnostic therapeutic approaches came in the form of lack of activity of BRAF inhibitors in colon cancers harboring BRAF V600E mutations. Ultimately, this disappointing clinical finding was elegantly explained by inadvertent activation of EGFR by BRAF inhibitors.1 Recent clinical trial data support this mechanism with triplet combination therapy of BRAF, MEK, and EGFR inhibition demonstrating improved activity.2 BRAF V600E mutations also occur in lung cancers. In an early example of lung cancer providing a testing ground for tumor-agnostic strategies, dabrafenib and trametinib demonstrated significant clinical activity with an objective response rate (ORR) of 63% and a durable median progression-free survival (mPFS) of 9.7 months, leading to European Medicines Agency approval in 2017 and later U.S. Food and Drug Administration (FDA) approval.3 Since this time, BRAF inhibitors with or without MEK inhibition have demonstrated clinical activity in hairy cell leukemia and anaplastic thyroid cancers harboring BRAF V600E mutations. Despite these successes across multiple tumor types, there is no tumor-agnostic approval yet for BRAF V600E mutations.
ALK
Oncogenic ALK gene fusions were first identified in anaplastic large cell lymphoma in 1994.4 However, the first clinical trial of an ALK inhibitor in cancer did not begin until after the discovery of ALK gene fusions in lung cancer in 2007.5 Currently there are five approved ALK inhibitors for NSCLC, but no tumor-agnostic approval despite the presence of ALK oncogenes in neuroblastoma, inflammatory myofibroblastic tumors, and many others.6 The My Pathway basket trial (NCT02091141) is evaluating the activity of alectinib in patients with ALK-positive tumors.
ROS1
ROS1 fusions were first identified in glioblastoma in 1987.7 The discovery of ROS1 fusions in lung cancer and the readily available ROS1 inhibitor crizotinib, which already had safety and efficacy data in ALK-positive NSCLC, facilitated rapid development of crizotinib as the first approved ROS1 inhibitor in ROS1-positive NSCLC.8 Similar to ALK research, ROS1 fusions have been identified in a number of other tumor types.9 The STARTRK-2 (NCT02568267, entrectinib) and AcSé (NCT02034981, crizotinib) basket trials are evaluating the role of ROS1 inhibitors in ROS1 fusion–positive tumors.
NTRK1/2/3
Gene fusions involving the NTRK1 gene, which encodes the TRKA receptor tyrosine kinase, were discovered in 1982 in a single colorectal cancer specimen10; however, NTRK1 gene fusions in lung cancer were first identified much later.11 Early preclinical data suggested that TRK inhibitors would have activity irrespective of tumor type and would target the related gene fusions involving NTRK2 (TRKB) and NTRK3 (TRKC), supportive of a tumor-agnostic therapeutic approach for this oncogene family.12 A review of the literature suggested that NTRK1/2/3 fusions occur across a number of tumor histologies, and given the relative rarity of these oncogenes overall,13 a basket trial design was pursued from the onset for TRK inhibitors. In 2018, larotrectinib was the first TRK inhibitor to be approved for the treatment of NTRK1/2/3 fusion–positive cancers. This was based on a cohort of 55 adult and pediatric patients representing 17 unique different histologies that harbored NTRK1/2/3 fusions.14 The ORR was 75% by independent review, and mPFS was not yet reached. Overall, the therapy was well tolerated with most common adverse events (AEs) being transaminitis, dizziness, fatigue, nausea, and notable increase in body weight, which may be an on-target effect of TRKB inhibition. This trial represented a number of firsts in oncology including the first oncogene-targeted, tumor-agnostic therapy to be approved (pembrolizumab for MSI-high tumors was the first tumor-agnostic therapy approved), the first cancer drug to be approved simultaneously for both adult and pediatric patients, and the first cancer drug to be approved for a family of oncogenes.
Entrectinib was the second agent to gain approval (Japan) for NTRK1/2/3 fusion–positive cancers. Entrectinib is an ALK/ROS1/TRK1 inhibitor designed to have activity in the central nervous system (CNS), an important feature for tumors with a high propensity for brain metastases, such as lung cancer. Integrated analysis from 54 patients with NTRK fusion–positive disease from ALKA-372-001 (EudraCT 2012-000148- 88), STARTRK-1 (NCT02097810), and STARTRK-2 (NCT02568267) included only adult patients with 19 different histologies represented.15 The ORR was 57%, and the mPFS was 11 months. The intracranial ORR (IC-ORR) in 11 patients with CNS metastases was similar to the overall ORR at 54.5%. Entrectinib was well tolerated; the most common AEs included dysgeusia, constipation, fatigue, dizziness, and weight gain. Analysis of 10 patients with NSCLC with NTRK1/3 fusions (no NTRK2 fusions were identified in NSCLC) demonstrated an ORR of 70%, median PFS of 14.9 months, and intracranial ORR of 66.7%.16 Notably, these results were similar to the entrectinib data for ROS1-positive NSCLC with an ORR of 77.4%, mPFS of 19.0 months, and IC-ORR of 55%.17 Thus, entrectinib has significant systemic and intracranial activity in both NTRK and ROS1 fusion–positive NSCLC.
Future Potential Tumor-Agnostic Targets
There are numerous potential tumor-agnostic indications on the horizon, all of which will likely include important driver oncogenes in lung cancer. RET gene fusions have been identified in lung cancer, papillary thyroid cancer, colorectal cancer, and other tumor types. There are ongoing studies of selective RET inhibitors for patients with cancers harboring RET fusions including selpercatinib (LOXO-292; LIBRETTO-001, NCT03157128) and pralsetinib (BLU-667; ARROW, NCT03037385). The FGFR1-4 inhibitor erdafitinib was recently FDA approved for urothelial carcinoma harboring prespecified FGFR alterations including FGFR fusions that have previously been identified in NSCLC and other cancers. Erdafitinib is being evaluated in tumors with FGFR fusions, mutations, or amplification in the National Cancer Institute MATCH study (NCT02465060). KRAS G12C mutations, which occur most frequently in NSCLC but are found in numerous other malignancies, represent another exciting tumor-agnostic opportunity; recently developed, mutation specific inhibitors such as MRTX849 (NCT03785249) and AMG 510 (NCT03600883), have demonstrated preliminary antitumor activity in NSCLC.18 Finally, gene fusions involving NRG1 were identified initially in NSCLC19 but recently have been identified in pancreatic, ovarian, and gallbladder cancers, among others.20
Conclusion
Tumor-agnostic therapeutic strategies represent the true embodiment of a precision medicine approach to cancer by specifically targeting biologically relevant pathways, irrespective of old tumor classification systems. Currently, NTRK fusions represent the only tumor-agnostic indication for targeted therapies in cancer, with the recent approvals of larotrectinib and entrectinib. These recent successes demonstrate the willingness for regulatory agencies to consider novel indications and provide a roadmap for future tumor-agnostic oncogene targets. Multiple opportunities still exist for new tumor-agnostic indications for other oncogenes in cancer, including ALK, ROS1, RET, FGFR, NRG1, KRAS G12C, and others. The future success of tumor-agnostic strategies will depend on several factors including the implementation of cross-tumor trial teams to facilitate enrollment beyond organ-specific tumor sites and, most importantly, the broad deployment of multiplexed next-generation sequencing panels to identify eligible patients. ✦
About the Author: Dr. Doebele is an associate professor of medicine at the University of Colorado Denver.
References:
1. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100-103.
2. Kopetz S, Grothey A, Van Cutsem E, et al. LBA-006 BEACON CRC: a randomized, 3-Arm, phase 3 study of encorafenib and cetuximab with or without binimetinib vs. choice of either irinotecan or FOLFIRI plus cetuximab in BRAF V600E– mutant metastatic colorectal cancer. Ann Oncol. 2019;30 (Suppl 4): iv137–iv151.
3. Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984-993.
4. Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281-1284.
5. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561-566.
6. Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2(6):495-502.
7. Birchmeier C, Sharma S, Wigler M. Expression and rearrangement of the ROS1 gene in human glioblastoma cells. Proc Natl Acad Sci U S A. 987;84(24):9270-9274.
8. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963-1971.
9. Davies KD, Doebele RC. Molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 2013;19(15):4040-4045.
10. Pulciani S, Santos E, Lauver AV, et al. Oncogenes in solid human tumours. Nature. 1982;300(5892):539-542.
11. Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19(11):1469-1472.
12. Doebele RC, Davis LE, Vaishnavi A, et al. An Oncogenic NTRK Fusion in a Patient with Soft -Tissue Sarcoma with Response to the Tropomyosin-Related Kinase Inhibitor LOXO- 101. Cancer Discov. 2015;5(10):1049-1057.
13. Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5(1):25-34.
14. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378(8):731-739.
15. Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and safety of entrectinib in patients with NTRK fusion-positive tumours: Pooled analysis of STARTRK-2, STARTRK-1, and ALKA-372- 001. Ann Oncol. 2018;29.
16. Doebele R, Paz-Ares L, Farago AF, et al. Abstract CT131: Entrectinib in NTRK-fusion positive non-small cell lung cancer (NSCLC): Integrated analysis of patients enrolled in three trials (STARTRK-2, STARTRK-1 and ALKA-372-001). Cancer Res. 2019;79:CT131.
17. Doebele R, Ahn M, Siena S, et al. OA02.01 Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion-Positive Non-Small Cell Lung Cancer (NSCLC). J of Thoracic Oncol. 2018;13:S321-S322.
18. Fakih M, O’Neil B, Price TJ, et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J Clin Oncol. 2019;37:3003-3003.
19. Fernandez-Cuesta L, Plenker D, Osada H, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014;4(4):415-422.
20. Jonna S, Feldman RA, Swensen J, et al. Detection of NRG1 Gene Fusions in Solid Tumors. Clin Cancer Res. 2019 Apr 15. [Epub ahead of print].