By Kara Nyberg, PhD
Posted: August 14, 2019
The 2019 Annual Meeting of the American Society of Clinical Oncology (ASCO) featured hundreds of lung cancer abstracts encompassing the full spectrum of this malignancy. This article highlights some of the most important NSCLC and SCLC research presented at ASCO that will help shape future areas of inquiry and, ultimately, real-life clinical practice.
Neoadjuvant Therapy in NSCLC
As immune checkpoint inhibitors become part of standard practice in advanced NSCLC, forays into earlier stages of disease offer new promise. Small pilot studies have indicated that immune checkpoint inhibitors might benefit patients with resectable NSCLC when used prior to surgery. “Upregulation of tumor PD-L1 has been shown to be critical for the spread and survival of lung metastasis in murine models of lung adenocarcinoma, supporting the testing of immune checkpoint inhibitors in the neoadjuvant setting to prime the intratumoral immune response and eradicate metastatic disease,” according to Tina Cascone, MD, PhD, of the University of Texas MD Anderson Cancer Center.
Two neoadjuvant studies presented at ASCO, LCMC3 and NEOSTAR, were designed to investigate these initial observations more thoroughly. LCMC3, presented by David J. Kwiatkowski, MD, PhD, of the Dana-Farber Cancer Institute, is an ongoing single-arm phase II trial evaluating two cycles of neoadjuvant atezolizumab in patients with stage IB to IIIB NSCLC (Abstract 8503). NEOSTAR, presented by Dr. Cascone, is a now-completed randomized phase II trial that assessed three cycles of neoadjuvant nivolumab, either alone or in combination with a single dose of ipilimumab, in patients with stage IA to IIIA NSCLC (Abstract 8504).
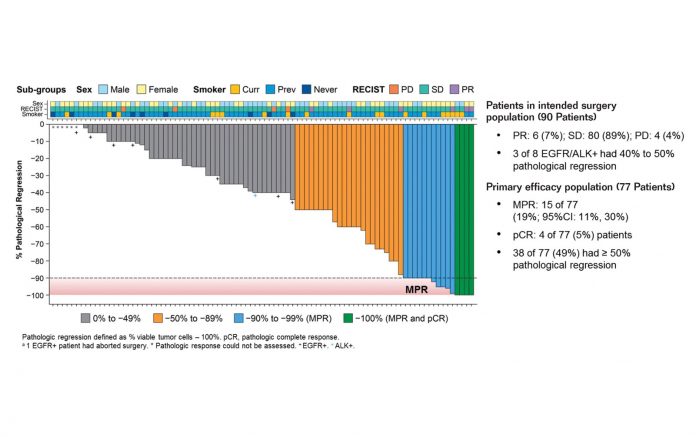
Neoadjuvant immunotherapy proved encouraging based on the primary efficacy endpoint of major pathologic response (MPR), defined as 10% or fewer viable tumor cells in the surgical resection specimen. In LCMC3, the MPR rate was 19% among 77 evaluable patients who received neoadjuvant atezolizumab (Fig. 1). In NEOSTAR, the MPR rate was 19% among 21 evaluable patients who received neoadjuvant nivolumab and 44% among 16 evaluable patients who received neoadjuvant nivolumab plus ipilimumab.
Each of these regimens was relatively well tolerated, with grade ≥ 3 treatmentrelated adverse events (TRAEs) occurring in only 6% to 13% of patients. Most patients were able to proceed to surgery (89% resection rate in both trials) due to low rates of disease progression (≤ 5%).
Maximillian Diehn, MD, PhD, of the Stanford Cancer Institute, who discussed the LCMC3 and NEOSTAR trials, acknowledged the favorable findings and noted that the MPR rates are consistent with those conferred by neoadjuvant multiagent chemotherapy. For example, in a study conducted in 41 patients with stage IB to IIIA NSCLC, four cycles of neoadjuvant cisplatin, docetaxel, and bevacizumab yielded an MPR rate of 27%.1
However, Dr. Diehn also emphasized that additional research will be needed to bolster the results. First, MPR has not been validated as a surrogate endpoint for OS, meaning that longer followup—preferably in the setting of larger randomized studies—will be required to determine whether neoadjuvant immunotherapy truly makes a difference in prolonging survival.
Second, both LCMC3 and NEOSTAR enrolled all-comers, potentially exposing some patients to unsuitable therapy. “I think we have a major unmet need for developing biomarkers for personalized treatment in this area,” Dr. Diehn remarked. Whereas both PD-L1 expression and tumor mutation burden (TMB) have been shown to independently predict the response to selected immunotherapy regimens in the metastatic setting (Abstract 9016), these biomarkers may not apply in the neoadjuvant setting or to all types of immune checkpoint inhibitors. In both LCMC3 and NEOSTAR, positive PD-L1 expression showed a significant but moderate correlation with MPR; in LCMC3, TMB and genes commonly mutated in NSCLC did not.
Finally, Dr. Diehn suggested that combined treatment with immunotherapy and chemotherapy may be much more effective in the neoadjuvant setting than either therapeutic class alone. Indeed, early results from small studies of carboplatin/paclitaxel combined with either nivolumab or atezolizumab have yielded MPR rates ranging from 64% to 80%.2,3 According to Dr. Diehn, this suggests that, “as in the advanced setting, the combination of immunotherapy and chemotherapy may be most active in [the neoadjuvant] setting,” particularly when patients are not selected based on predictive biomarkers. In fact, four separate phase III studies of neoadjuvant immunotherapy in combination with a platinum doublet are currently underway to help address this very issue: CheckMate 816 with nivolumab (NCT02998528), KEYNOTE-617 with pembrolizumab (NCT03425643), IMpower030 with atezolizumab (NCT03456063), and AEGEAN with durvalumab (NCT03800134).
Definitive Concurrent Chemoradiation in NSCLC
Improving on definitive platinum-based doublet chemoradiotherapy for patients with unresectable stage III NSCLC remains elusive in light of negative results from NRG-LU001, a randomized phase II trial that failed to show improved outcomes with the addition of metformin to concurrent chemoradiotherapy (Abstract 8502). The rationale for NRG-LU001 was sound: Although metformin is a well-established diabetes medication that influences glucose metabolism, the agent has also been found to activate tumor-suppressing pathways and enhance the response to radiotherapy and chemotherapy in preclinical NSCLC models. Unfortunately, metformin failed to deliver when tested in humans based on the NRG-LU001 data presented by Theodoros Tsakiridis, MD, PhD, of McMaster University.
“As in the advanced setting, the combination of immunotherapy and chemotherapy may be most active in [the neoadjuvant] setting, particularly when patients are not selected based on predictive biomarkers.” –Dr. Maximillian Diehn
NRG-LU001 included 167 patients with inoperable stage IIIA/IIIB NSCLC but without comorbid diabetes. Participants were stratified by performance status, disease histology, and clinical stage and randomly assigned to standard concurrent chemoradiotherapy (carboplatin/ paclitaxel + full-dose radiotherapy) either alone or combined with concurrent metformin. In both arms, concurrent chemoradiotherapy was administered for 6 weeks, followed by 6 weeks of consolidation chemotherapy (plus metformin in the investigational arm).
NRG-LU001 did not meet the primary endpoint of improved PFS with the addition of metformin to chemoradiotherapy. Median PFS in the intention-to-treat population reached 12.2 months in the group that received metformin compared with 16.6 months for the group that did not (HR: 1.15; 95% CI: 0.77-1.73; p = 0.2441). The addition of metformin to chemoradiotherapy also did not improve median OS (40.1 vs 38.5 months; HR: 1.03; 95% CI: 0.64-1.68; p = 0.8910), the median time to locoregional failure (HR: 0.91; 95% CI: 0.51-1.62), or the median time to distant failure (HR: 1.29; 95% CI: 0.71-2.34).
“It should be noted that in this study, we observed higher-than-expected survival outcomes compared to recently reported phase III trials,” Dr. Tsakiridis remarked.
First-Line Treatment of Metastatic NSCLC
In metastatic NSCLC, two randomized phase III trials—RELAY and a study conducted at the Tata Memorial Hospital in India—assessed whether augmenting targeted therapy with antiangiogenic therapy or cytotoxic chemotherapy, respectively, could improve outcomes in the first-line setting for patients with EGFR-mutated disease.
Although dual blockade of the EGFR and VEGF pathways might lead to synergistic antitumor activity, Japanese trials that have evaluated this approach using a first-generation EGFR tyrosine kinase inhibitor (TKI) in combination with bevacizumab (ie, JO25567, NEJ026) have yielded mixed results.4,5 The RELAY trial (Abstract 9000) differed from these prior studies in that (a) it was a global study conducted at multiple sites in Asia, Europe, and North America and (b) it featured ramucirumab, which targets VEGF receptor 2, instead of bevacizumab, which targets VEGF ligands.
RELAY included 449 patients with stage IV NSCLC harboring common, actionable EGFR mutations (exon 19 deletion or exon 21 L858R); they were randomly assigned to receive erlotinib plus ramucirumab (ER) or erlotinib plus placebo (EP). The trial met the primary endpoint by demonstrating a significant 7-month improvement in median PFS with the addition of ramucirumab to erlotinib, compared to placebo (19.4 vs 12.4 months; HR: 0.591, 95% CI: 0.461- 0.760; p < 0.0001). The improvement in PFS appeared to be driven by a prolonged duration of response for ER and EP, respectively (18.0 vs 11.1 months) rather than an improvement in response rate (76% vs 75%). Moreover, the PFS benefit conferred by ramucirumab may also extend to OS (HR: 0.832, 95% CI: 0.532-1.303), although the data are not yet fully mature.
In terms of toxicity, ramucirumab led to a higher rate of grade ≥ 3 TRAEs when added to erlotinib compared with placebo (72% vs 54%); however, discontinuation rates due to TRAEs were similar for the respective arms (13% vs 11%). The principal toxicity associated with ramucirumab was hypertension (all grades: 45% vs 12% with placebo; grade 3: 24% vs 5% with placebo).
The phase III trial conducted at the Tata Memorial Hospital assessed whether adding pemetrexed/carboplatin to gefitinib improved median PFS in 350 patients with EGFR-mutated, unresectable stage IIIB or stage IV NSCLC (Abstract 9001). Eligibility for this trial was less stringent and included patients with an Eastern Cooperative Oncology Group performance status of 0 to 2; activating EGFR mutations in exon 19, 21, or 18; and it permitted individuals with brain metastases.
Like RELAY, the Tata Memorial trial successfully documented a significant improvement in PFS, the primary endpoint, with gefitinib plus chemotherapy versus gefitinib alone (16.0 vs 8.0 months; HR: 0.51, 95% CI: 0.39-0.66; p < 0.0001). However, unlike RELAY, this improvement was driven by a higher response rate (75% vs 63%; p = 0.01) and depth of response (median tumor size reduction: 56.4% vs 43.5%; p = 0.002). The study also demonstrated a significant improvement in median OS with the addition of pemetrexed/carboplatin to gefitinib versus gefitinib alone (not reached vs 17.0 months; HR: 0.45, 95% CI: 0.31-0.65; p < 0.0001).
As expected, adding chemotherapy to gefitinib increased toxicity in comparison to gefitinib alone. Clinically relevant grade ≥ 3 adverse events occurred in 51% in the investigational arm versus 25% in the control arm, largely driven by increases in hematologic events. Additionally, 17% of patients in the investigational arm discontinued pemetrexed due to toxicity, whereas the rate of gefitinib discontinuation in either arm was ≤ 1%.
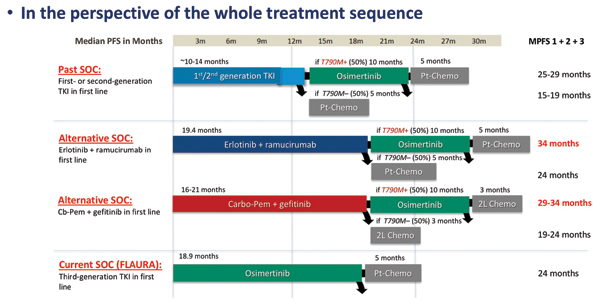
As Maurice Pérol, MD, of the Centre Léon Bérard, remarked during his discussion of these two studies, a key shortcoming of both trials is the fact that the investigational regimens were not compared against osimertinib, a third-generation EGFR TKI that represents a new standard of care for EGFR-mutated disease in many parts of the world. Acknowledging the caveats of cross-trial comparisons, Dr. Pérol deduced that in the first-line setting, adding either ramucirumab or pemetrexed/carboplatin to a first-generation TKI probably yields about the same PFS duration as osimertinib alone. However, in terms of treatment sequencing, using one of the novel regimens up front and reserving osimertinib for patients with T790M-positive disease at the time of progression may lead to better survival outcomes across all lines of therapy (Fig. 2). Ultimately, the preferred first-line treatment for EGFR-mutated NSCLC will depend on patient characteristics, disease characteristics (eg, brain metastasis, co-occurring mutations), the tolerability profile of a given regimen, patient-reported outcomes, cost, and treatment availability, according to Dr. Pérol.
Maintenance Treatment of Metastatic NSCLC
Looking beyond first-line therapy, a randomized phase III clinical trial, ECOG-ACRIN 5508, evaluated which maintenance regimen should be the standard of care when bevacizumab is included as part of the induction regimen in patients with advanced nonsquamous NSCLC (Abstract 9002). More specifically, three maintenance regimens—bevacizumab monotherapy, pemetrexed monotherapy, and bevacizumab plus pemetrexed combination therapy—were compared among 1,516 patients who achieved stable disease or better following four cycles of firstline carboplatin/paclitaxel plus bevacizumab. The participants were stratified by smoking status, sex, disease stage, and response to induction therapy prior to randomization, but not by EGFR/ALK mutation status since the trial was designed in 2010 before such testing became part of routine practice.
“Ultimately, the preferred first-line treatment for EGFR-mutated NSCLC will depend on patient characteristics, disease characteristics (eg, brain metastasis, co-occurring mutations), the tolerability profile of a given regimen, patient-reported outcomes, cost, and treatment availability” –Dr. Maurice Pérol
The findings showed that the addition of pemetrexed to bevacizumab improved median PFS versus both bevacizumab monotherapy and pemetrexed monotherapy (7.5 vs 4.2 and 5.1 months, respectively; p < 0.001 for bevacizumab + pemetrexed vs bevacizumab monotherapy vs pemetrexed monotherapy). However, this did not translate into increased median OS (16.4 vs 14.4 and 15.9 months; p = 0.28 for bevacizumab + pemetrexed vs bevacizumab monotherapy), which was the primary endpoint of the trial. Combination maintenance also resulted in a higher incidence of grade 3/4 TRAEs compared with either bevacizumab or pemetrexed alone (50% vs 29% and 37%, respectively).
Given three clinical trials—ECOGACRIN 5508, AVAPERL,6 and COMPASS7—that have now failed to show an OS benefit with bevacizumab-pemetrexed maintenance therapy following prior bevacizumab-containing therapy, Dr. Pérol concluded that “we do not have any clear evidence, to date, [justifying] combination maintenance for our patients…. Pemetrexed is still the preferred maintenance option after a pemetrexed-containing induction regimen.” He noted that in situations where pemetrexed is not used up front, such as after carboplatin/paclitaxel/bevacizumab induction therapy, maintenance bevacizumab constitutes an acceptable alternative that offers a lower level of toxicity.
Novel Cytotoxic Therapy for SCLC
Tremendous unmet clinical need exists in SCLC, and yet no novel therapies have been able to top second-line topotecan. “Although topotecan leaves much to be desired with respect to both efficacy and toxicity, it is the standard of care, and no investigational agent has shown superiority in a randomized study over the past 20 years,” according to Anna F. Farago, MD, PhD, of Massachusetts General Hospital and Harvard Medical School.
Lurbinectedin, a synthetic analog of trabectedin administered intravenously every 3 weeks, may prove worthy of challenging topotecan’s position. Luis Paz Ares, MD, PhD, of the Hospital Universitario, presented the results from a phase II trial of single-agent lurbinectedin conducted in patients with SCLC whose disease had progressed after one prior line of chemotherapy with or without immunotherapy (Abstract 8506).
As Dr. Paz Ares explained, SCLC is a transcription-addicted tumor driven by dysregulated expression of several key transcription factors. Lurbinectedin upsets these processes by binding to gene promoter regions, creating DNA breaks, and inhibiting transcription, ultimately downregulating the expression of growth-promoting proteins.
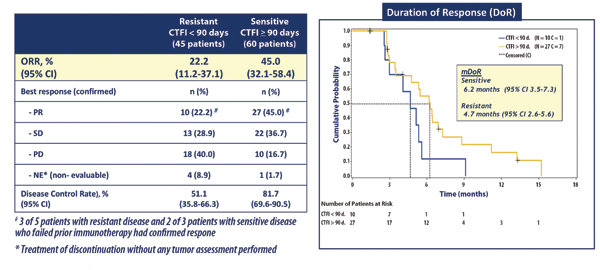
Among the 105 patients included in the trial, the objective response rate (ORR) to lurbinectedin was 35.2%— all partial responses—and the median duration of response was 5.3 months. Of note, 5 of 8 patients who failed prior immunotherapy demonstrated a response to lurbinectedin.
Subgroup analyses revealed that lurbinectedin conferred activity regardless of whether patients had platinum-sensitive or platinum-resistant disease (Fig. 3 ). Moreover, about 40% of patients attained a longer PFS duration with lurbinectedin than with their first-line chemotherapy. The median PFS with lurbinectedin monotherapy was 3.9 months, and median OS was 9.3 months.
Lurbinectedin appeared to be relatively well tolerated, with a manageable safety profile. The most common all-grade TRAEs included fatigue (58.1%), nausea (32.4%), and decreased appetite (21.0%), the great majority of which were mild or moderate in severity. By far the most common grade 3/4 TRAE was neutropenia (22.9%). Few patients discontinued treatment due to adverse events (1.9%) or experienced treatment-related serious adverse events (10.5%), and there were no treatment-associated deaths.
“Although topotecan leaves much to be desired with respect to both efficacy and toxicity, it is the standard of care, and no investigational agent has shown superiority in a randomized study over the past 20 years.” –Dr. Anna F. Farago
“We may conclude lurbinectedin is emerging as a potential new treatment alternative for the second-line setting in [patients with] SCLC,” Dr. Paz Ares remarked.
Dr. Farago affirmed that lurbinectedin edges out topotecan based on historical efficacy data given numerically better ORR and OS findings. However, she noted that the “high OS that we see with lurbinectedin may in part reflect the activity of this drug, but may also reflect the trend that we’ve seen over time with OS improving in SCLC studies in the second-line space.”
Although lurbinectedin has received an Orphan Drug Designation by the U.S. Food and Drug Administration for the treatment of SCLC, Dr. Farago feels it will be important to see phase III data for the agent. This will come from the global, randomized phase III ATLANTIS study that will compare lurbinectedin plus doxorubicin versus either topotecan or cyclophosphamide/ doxorubicin/vincristine in patients with SCLC progression following one line of platinum-based chemotherapy (NCT02566993). ✦
References
1. Chaft JE, Rusch V, Ginsberg MS, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. J Thorac Oncol. 2013;8(8):1084-1090.
2. Provencio M, Nadal E, Insa A, et al. Phase II study of neo-adjuvant chemo/immunotherapy for resectable stages IIIA non-small cell lung cancer— NADIM Study-SLCG. Presented at the 19th World Conference on Lung Cancer; Toronto, Canada; September 23-26, 2018. Abstract OA01.05.
3. Shu CA, Grigg C, Chiuzan C, et al. Neoadjuvant atezolizumab + chemotherapy in resectable nonsmall cell lung cancer (NSCLC). Presented at the 2018 ASCO Annual Meeting; Chicago, Illinois; June 1-5, 2018. Abstract 8532.
4. Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236-1244.
5. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced nonsquamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625-635.
6. Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044-1052.
7. Seto T, Azuma K, Yamanaka T, et al. A randomized phase III study of continuous maintenance bevacizumab with or without pemetrexed after induction therapy with carboplatin (Car), pemetrexed (Pem), and bevacizumab (Bev) for advanced non-squamous non-small cell lung cancer (nSQ-NSCLC) without sensitizing EGFR mutations: The COMPASS study (WJOG5610L). Presented at the 2019 ASCO Annual Meeting; Chicago, Illinois; May 31-June 4, 2019. Abstract 9003.