Abbreviations: A, ADI-PEG20; ADI-PEG20, arginine-lowering agent pegylated arginine deiminase; AR, Anetumab ravtansine; Beva, bevacizumab; BSC, best supportive care; NA, not applicable; NR, not reported; n.s. not significant; V, vinorelbine; MOA, Mechanism of action.
By Cornedine Jannette de Gooijer, MD; Maria Disselhorst, MD; and Paul Baas, MD, PhD
Posted: May 27, 2019

Until 2015, few changes occurred with respect to available therapies for malignant pleural mesothelioma (MPM). Luckily, a renewed interest in MPM with emphasis on immunotherapy has led to several phase II and III trials. The latest results and running trials in MPM are presented in the tables and are discussed in more detail in the article.
Surgery
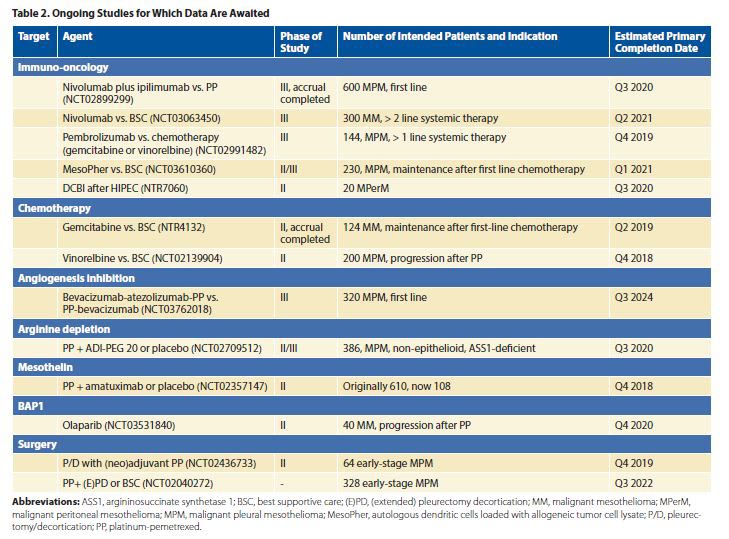
Whether to offer surgery to patients with MPM is subject to physicians’ preferences and experience. The MARS 2 trial in the United Kingdom will evaluate the additional value of (extended) pleurectomy/decortication (P/D) to chemotherapy (NCT02040272). In this trial, 328 patients with MPM will be randomly assigned to chemotherapy with or without extended P/D. In the EORTC- 1205 trial, the timing of chemotherapy (before or after) P/D will be examined in 64 patients (NCT02436733). Patients with limited-disease malignant peritoneal mesothelioma (MPerM) can participate in the MESOPEC study, in which adjuvant dendritic cell–based immunotherapy after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy is being tested.

Chemotherapy
Evidence for maintenance chemotherapy in MPM is lacking. Maintenance pemetrexed has been under investigation since 2010, but no results have been published to date (NCT01085630). In the Netherlands, a study of switch-maintenance therapy with gemcitabine versus observation in the first-line setting in patients with nonprogressing disease after platinum/ pemetrexed has finished accrual, and the first results are expected this year. To date, there is still no approved second-line treatment option. Retreatment with pemetrexed (plus platinum) can be considered.1 Vinorelbine is under investigation in two randomized phase II studies, one comparing active symptom control to active symptom control with vinorelbine (NCT02139904) and the other comparing pembrolizumab to gemcitabine or vinorelbine (NCT02991482).

Angiogenesis Inhibitors
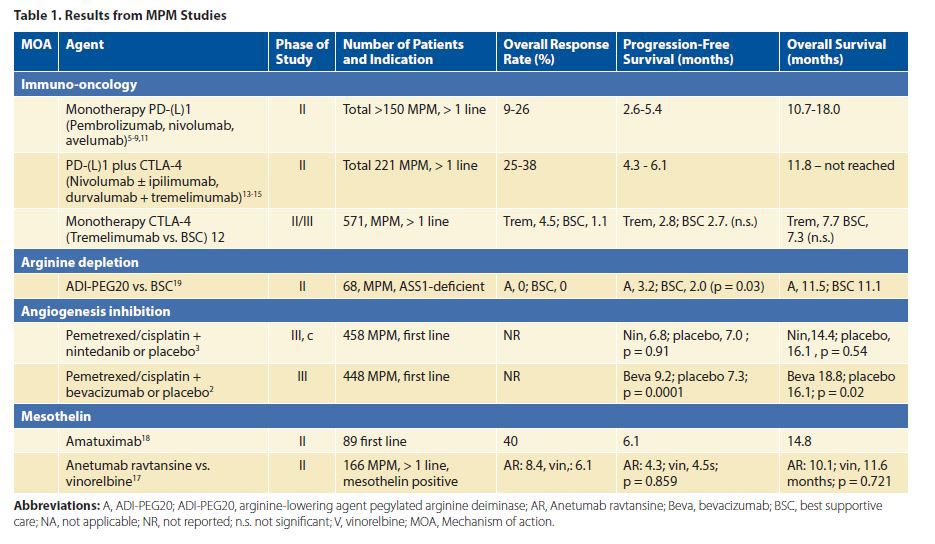
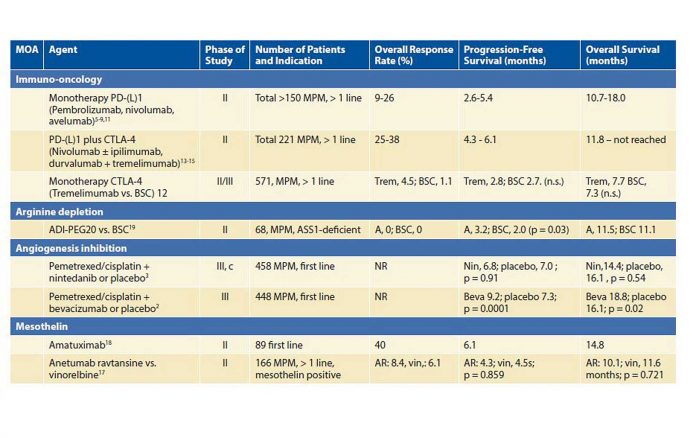
Bevacizumab is the only angiogenesis inhibitor that has demonstrated a benefit in the management of MPM in phase III testing. It yielded a 2.7 month survival benefit in combination with first-line chemotherapy (pemetrexed/cisplatin) versus chemotherapy alone in 448 patients with MPM.2 The addition of bevacizumab to chemotherapy is now considered a treatment option.1 Nintedanib, an antiangiogenic tyrosine kinase inhibitor, showed promise in the phase II setting but failed to improve progression-free survival in combination with first-line chemotherapy in a phase III trial.3,4
Immunotherapy
Over the past few years, multiple promising phase II trials have been published on immune checkpoint inhibitors for MPM in the second or later line. Monotherapy PD-1 inhibitors (e.g., nivolumab and pembrolizumab), have yielded objective response rates (ORRs) of approximately 20%.5-10 The PD-L1 checkpoint inhibitor avelumab had a somewhat lower ORR of 9%.11 The only randomized phase IIB trial of immunotherapy with monotherapy tremelimumab (a CTLA-4 inhibitor) versus placebo failed to show any benefit.12 Recently, two phase II trials testing nivolumab plus ipilimumab13,14 and a trial testing tremelimumab plus durvalumab showed objective response rates between 25% and 38%.15
In the first line, a phase III study randomly selecting between standard chemotherapy and nivolumab plus ipilimumab is ongoing, and initial results are expected this year (NCT02899299). In further lines, a monotherapy PD-1 inhibitor is being tested in two phase III trials, versus either best supportive care (NCT03063450) or chemotherapy (NCT02991482).
Dendritic cell immunotherapy has attracted attention for its activity in mice and humans. Autologous monocyte–derived dendritic cells are pulsed with tumor lysate from five different mesothelioma cell lines and reintroduced into the patient.16 This led to a phase II/III trial testing maintenance vaccination versus observation after effective first-line chemotherapy (NCT03610360).
Targeted Therapy
Mesothelin is a tumor antigen that is highly expressed in epithelioid MPM, which makes it an interesting target for therapies. Anetumab ravtansine is an antibody drug conjugate that is bound and internalized by mesothelin-expressing tumor cells. Unfortunately, compared to vinorelbine, it failed to improve progression-free survival.17 A trial with pembrolizumab combined with anetumab ravtansine is recruiting patients in the United States (NCT03126630). Amatuximab is a chimeric monoclonal immunoglobulin antibody targeting mesothelin. In a multicenter phase II study, amatuximab in combination with pemetrexed and cisplatin was well tolerated and resulted in a disease control rate of 90%. However, the primary endpoint, 3-month improvement in progression-free survival, was not significant.18
Argininosuccinate synthase 1 is absent in up to 63% of MPM, making these cells dependent on systemic arginine. When cells are depleted of arginine, they go into apoptosis. Pegylated arginine deiminase (ADI-PEG) depletes systemic arginine. Single-agent ADI-PEG in 68 patients with ASS1-deficient MPM (out of 201 screened patients) resulted in a small progression-free-survival benefit and no overall survival benefit.19 The ongoing ATOMIC-Meso phase II/III trial randomly assigns patients with mixed-type and sarcomatoid MPM to pemetrexed and cisplatin plus either ADI-PEG or placebo (NCT02709512).
BRCA-associated protein 1 (BAP1) is one of the most common somatic mutations in MPM. This tumor suppressor is critical in repairing double-strand DNA breaks. Olaparib, an inhibitor of poly ADP ribose polymerase (PARP), an enzyme involved in DNA repair, will be tested in 40 patients with MPM (NCT03531840).
Another strategy to target DNA repair is to block enhancer of zeste-homolog 2 (EZH2), which is often upregulated when BAP1 is mutated. EZH2 regulates differentiation of stem and progenitor cells. Preliminary results with tazemetostat, an EZH2 inhibitor, demonstrated a disease control rate of 51% at 12 weeks; 15 patients (25%) sustained disease control at 24 weeks, 5 of whom continue to have ongoing disease control.20 ✦
About the Authors: Dr. de Gooijer is with the department of Thoracic Oncology, The Netherlands Cancer Institute, Amsterdam, the Netherlands. Dr. Disselhorst is with the department of Thoracic Oncology, The Netherlands Cancer Institute, Amsterdam, the Netherlands. Dr. Baas is chief of the Department of Thoracic Oncology, Netherlands Cancer Institute, Amsterdam, the Netherlands.
References:
1. Malignant Pleural Mesothelioma. National Comprehensive Cancer Network. www2.tri-kobe.org/nccn/guideline/lung/english/mpm.pdf. Published February 26, 2018. Accessed January 23, 2019.
2. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405-1414.
3. Scagliotti G, Gaafar R, Nowak A, et al. PL02.09 Nintedanib + Pemetrexed/Cisplatin in Patients with Unresectable MPM: Phase III Results from the LUME-Meso Trial. J Thorac Oncol. 2018;13:S186.
4. Grosso F, Steele N, Novello S, et al. Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial. J Clin Oncol. 2017;35(31):3591-3600.
5. Quispel-Janssen J1, van der Noort V2, de Vries JF, et al. PD-1 blockade with nivolumab in patients with recurrent Malignant Pleural Mesothelioma. J Thorac Oncol. 2018;13(10):1569-1576.
6. Goto Y, Okada M, Kijima T, et al. MA 19.01 A Phase II Study of Nivolumab: A Multicenter, Open-Label, Single Arm Study in Malignant Pleural Mesothelioma (MERIT). J Thorac Oncol. 2017;12:S1883.
7. Scherpereel A, Mazieres J, Greillier L, et al. Second- or third-line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: Results of the IFCT-1501 MAPS2 randomized phase II trial. J Clin Oncol. 2017;35(18):LBA8507-LBA8507.
8. Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623-630.
9. Desai A, Karrison T, Rose R, et al. Phase II trial of pembrolizumab (P) in patients (pts) with previously- treated mesothelioma (MM). J Clin Oncol. 2018;36:8565-8565.
10. Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J Thorac Oncol. 2018;13(10):1569-1576.
11. Hassan R, Thomas A, Nemunaitis JJ, et al. Efficacy and Safety of Avelumab Treatment in Patients With Advanced Unresectable Mesothelioma: Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019 Jan 3. [Epub ahead of print].
12 Maio M, Scherpereel A, Calabrò L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18:1261-1273.
13. Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, openlabel, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019 Jan 16. [Epub ahead of print].
14. Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019 Jan 16. [Epub ahead of print].
15. Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med. 2018;6(6):451-460.
16. Aerts JGJV, de Goeje PL, Cornelissen R, et al. Autologous Dendritic Cells Pulsed with Allogeneic Tumor Cell Lysate in Mesothelioma: From Mouse to Human. Clin Cancer Res. 2018;24:766-776.
17. Novello S, Blumenschein G, Bearz A, et al. Randomized Phase II Study of Anetumab Ravtansine or Vinorelbine in Patients with Malignant Pleural Mesothelioma. Presented at: International Association for the Study of Lung Cancer 18th World Conference on Lung Cancer; Yokohama, Japan: October 15-18, 2017. Abstract OA 02.01.
18. Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res. 2014;20:5927-5936.
19. Szlosarek PW, Baas P, Ceresoli GL, et al. ATOMIC-Meso: A randomized phase 2/3 trial of ADI-PEG20 or placebo with pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient non-epithelioid mesothelioma. J Clin Oncol. 2017;35:TPS8582-TPS8582.
20. Zauderer MG, Szlosarek P, Le Moulec S, et al. Phase 2, multicenter study of the EZH2 inhibitor tazemetostat as monotherapy in adults with relapsed or refractory (R/R) malignant mesothelioma (MM) with BAP1 inactivation. J Clin Oncol. 2018;36:8515-8515.
21. Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med. 2019;7(3):260- 270.