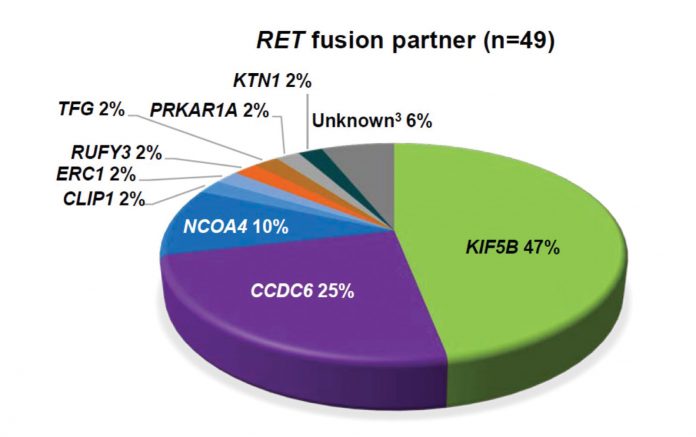
Reproduced with permission from Drilon A, et al.
By Karen L. Reckamp, MD, MS
Posted: March 1, 2019

Identification of genetic alterations in NSCLC has transformed diagnosis and treatment for patients with advanced disease. Identification of activating mutations in the EGFR gene in 2004 started the shift in our understanding of the complex biology of molecular subtypes of lung cancer. Genetic alterations continue to be described, and approximately 60% of patients with the adenocarcinoma subtype of NSCLC have a defined molecular alteration that may be amenable to targeted therapy.1 Emerging targets include the MET gene, through gene amplification and exon 14 skipping mutations; RET gene alterations, which occur in several tumor types; HER2, which can be amplified or mutated in NSCLC; and NTRK alterations, which are found in many tumor types, including NSCLC. These less-common alterations and promising treatments will be discussed.
MET
MET is a proto-oncogene that encodes for the transmembrane MET tyrosine receptor kinase. Through ligand binding to hepatocyte growth factor, signaling pathways such as PI3K/AKT, MAPK, NF-kB, and signal transducer and activator of transcription proteins (STATs) are activated. This leads to cell proliferation and invasion. Protein over-expression and phosphorylation are the most common forms of MET-positive NSCLC, but responses to therapy have been varied. MET amplification occurs in less than 5% of lung adenocarcinoma. MET exon 14 alterations are found in up to 4% of lung adenocarcinomas and are predominantly associated with older age (median age of 73 years) and significant smoking history.2 MET gene rearrangement is uncommon, but the kinase fusion KIF5B-MET has been reported in lung adenocarcinoma.3
Multitargeted tyrosine kinase inhibitors (TKIs) have been used to target MET in lung cancer (e.g., cabozantinib, crizotinib, and merestinib), and a variety of TKIs with increased MET sensitivity are also under investigation (e.g., savolitinib, tepotinib, capmatinib, SAR125844, sitravatinib, AMG 337, and tivantinib). In addition, monoclonal antibodies are being evaluated for patients with MET-driven tumors. In patients with MET exon 14 mutations and MET amplification, treatment with crizotinib (a dual MET/ALK inhibitor) has led to antitumor responses.4,5 In addition, cabozantinib has demonstrated tumor response in patients with exon 14 mutations.6 A phase I study was conducted to assess the safety and efficacy of crizotinib for patients with MET amplification. Patients with high levels of MET amplification (MET/CEP7 4) demonstrated antitumor activity, with a median PFS of 6.7 months.7 Furthermore, a phase II study of tepotinib in patients with MET exon 14 skipping mutations is ongoing, and available data for 22 treated patients were presented.8 Partial response was seen in 60% by investigator review; the most common toxicities included edema and diarrhea.
MET amplification has been identified in 5% to 20% after treatment with an EGFR TKI therapy (e.g., erlotinib, gefitinib, or osimertinib),9,10 and this has been described as an alternative mechanism of resistance to EGFR TKIs in patients with EGFR mutation–positive NSCLC, leading to ERBB3 signaling.11 Combination MET and EGFR TKI therapy is being studied as a treatment option for patients with resistant EGFR-mutant NSCLC.
RET
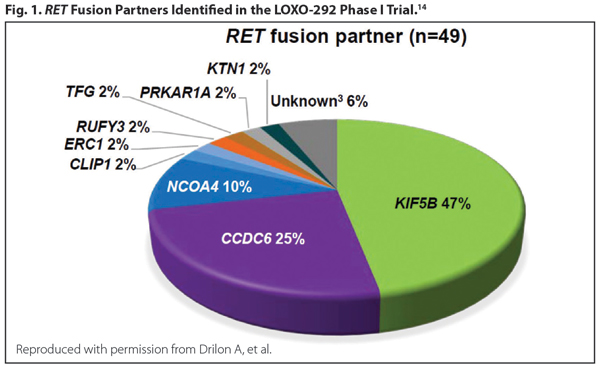
RET is a receptor tyrosine kinase that induces cellular proliferation, migration, and differentiation when activated. RET fusions occur in 1.4% of NSCLC and are more likely to be present in younger, never-smoking patients with adenocarcinoma. Clinical data from a phase II trial on the use of cabozantinib for patients with RET fusion–positive disease revealed two patients with partial responses,12 and the final results in 25 patients with RET-positive disease revealed an overall response rate (ORR) of 28%.13 The results from a global, multicenter registry of 165 patients with RET-positive disease from Europe, Asia, and the United States were reported.14 Of note, the ORR to cabozantinib, vandetanib, and sunitinib was detailed as 37%, 18%, and 22%, respectively. The median progression-free survival (PFS) was 2.3 months, and median overall survival (OS) was 6.8 months in all patients. On the other hand, newer agents look more promising. The novel RET inhibitor LOXO-292 yielded an ORR of more than 70% in RET-altered NSCLC and was well tolerated, with most adverse events grade 1 (Fig. 1).15 BLU-667, another potent and selective RET inhibitor, has also demonstrated preclinical activity and clinical responses in patients with RET-altered NSCLC.16
HER2
HER2 is a member of the erbB receptor tyrosine kinase family. It has no known ligand and is activated by homodimerization or heterodimerization with other members of the erbB family. It activates signaling through the PI3K-AKT and MEK-ERK pathways. HER2 amplification occurs infrequently, and exon 20 insertion mutations in HER2 are seen in approximately 2% of NSCLC.17
Poziotinib has been identified as a novel, potent inhibitor of HER2 exon mutations with tumor responses in a phase II trial with 11 patients.18 In a phase II basket trial, 18 patients with HER2-mutant lung adenocarcinomas were treated with ado-trastuzumab emtansine (T-DM1). This was the first positive trial evaluating T-DM1 in patients with HER2-mutated lung cancer; the partial response rate was 44%, and median PFS was 5 months.19 A retrospective study assessed 101 patients treated with chemotherapy or HER2-targeted therapy.20 The median overall survival was 24 months for all patients regardless of therapy received. Sixty-five patients received HER2-targeted therapy (i.e., trastuzumab, neratinib, afatinib, lapatinib, or T-DM1), and ORR was highest at 50.9% for those who received trastuzumab with or without chemotherapy or T-DM1, with PFS 4.8 months. A phase II trial investigated dacomitinib (a pan-HER inhibitor) in 30 patients with HER2-mutant or amplified NSCLC and resulted in a 12% ORR.21
NTRK
Tropomyosin-related kinase (TRK) encodes the tyrosine kinase receptors for neurotrophins associated with the nerve growth factor (NGF) family.22 Three members of this family include the NTRK1, NTRK2, and NTRK3 proto-oncogenes. Fewer than 1% of NSCLC cases have NTRK fusions.23 NTRK fusions can be found in both men and women with wide ranges of age and smoking history.24
A phase I study of entrectinib demonstrated antitumor activity in one patient with a NTRK1 fusion.25 In combined phase I/II trials evaluating larotrectinib in patients across tumor types and age groups who were TRK positive, 55 patients were enrolled.26 The study demonstrated an ORR of 75% based on independent review. In addition, 71% of responses were ongoing, and 55% patients remained progression free at 1 year, strongly suggesting substantial activity in patients with NTRK fusion–positive disease. Additional NTRK inhibitors are also in development.
Future Directions
Targeted therapies have led to improved outcomes for patients with lung cancer, and additional targets and treatments continue to emerge. Genomic sequencing using broad platforms and bloodbased cell-free DNA have illuminated the intricacies of lung cancer. Understanding tumor biology helps to advance precision treatment to improve patient outcomes. Despite this progress, resistance invariably develops, resulting in even more complex and heterogeneous tumors. Further research is needed to understand mechanisms of resistance to prolong survival and quality of life. ✦
About the Author: Dr. Reckamp is a medical oncologist at the City of Hope Comprehensive Cancer Center.
References:
1. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014; 311:1998-2006.
2. Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplifi cation and c-Met Overexpression. J Clin Oncol. 2016;34(7):721-730.
3. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846.
4. Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymalepithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942-946.
5. Mendenhall MA, Goldman JW. MET-Mutated NSCLC with Major Response to Crizotinib. J Thorac Oncol. 2015;10(5):e33-34.
6. Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5(8):842-849.
7. Camidge DR, Otterson GA, Clark JW, et al. Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): Updated safety and efficacy findings from a phase 1 trial. J Clin Oncol. 2018;36(15):(suppl; abstr 9062).
8. Felip E, Horn L, Patel JD, et al. Tepotinib in patients with advanced non-small cell lung cancer harboring MET exon 14-skipping mutations: Phase II trial. J Clin Oncol. 2018;36(15):(suppl; abstr 9016).
9. Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932-20937.
10. Piotrowska Z, Thress KS, Mooradian M, et al. MET amplification as a resistance mechanism to osimertinib. J Clin Oncol. 2017;35(15):(suppl; abstr 9020).
11. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039-1043.
12. Drilon A, Wang L, Hasanovic A, et al. Response to Cabozantinib in patients with RET fusion-positive lung adenocarcinomas. Cancer Discov. 2013(6);3:630-635.
13. Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RETrearranged non-small-cell lung cancer: an openlabel, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016;17(12):1653-1660.
14. Gautschi O, Milia J, Filleron T, et al. Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol. 2017;35(13):1403-1410.
15. Drilon AE, Subbiah V, Oxnard GR, et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J Clin Oncol. 2018;36:(suppl; abs 102).
16. Subbiah V, Gainor JF, Rahal R, et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov. 2018;8(7):836-849.
17. Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65(5):1642-1646.
18. Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20–selective kinase inhibitor in non–small cell lung cancer. Nat Med. 2018;24(5):638- 646.
19. Li BT, Shen R, Buonocore D, et al. Ado-Trastuzumab Emtansine for Patients With HER2- Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol. 2018;36(24):2532-2537.
20. Mazieres J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Annals Oncol. 2016;27(2):281-286.
21. Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26(7):1421-1427.
22. Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169(2):107-114.
23. Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19(11):1469-1472.
24. Farago AF, Taylor MS, Doebele RC, et al. Clinicopathologic features of non-small cell lung cancer (NSCLC) harboring an NTRK gene fusion. JCO Precis Oncol. 2018 Jul 23 [Epub ahead of print].
25. Farago AF, Le LP, Zheng Z, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol. 2015;10(12):1670-1674.
26. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378(8):731-739.










