Posted: March 1, 2019
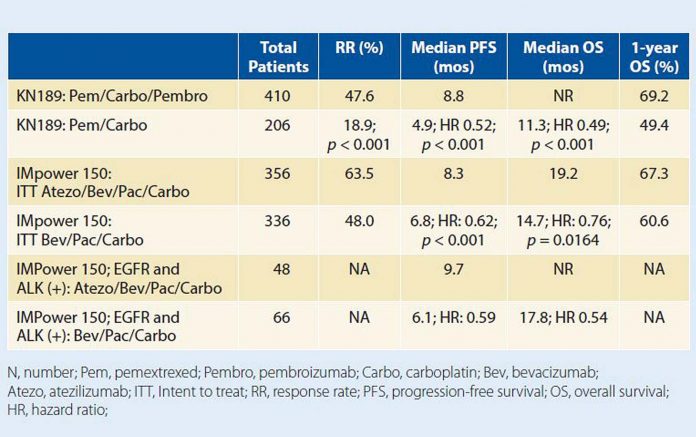
On December 6, 2018, the U.S. Food and Drug Administration (FDA) approved atezolizumab in combination with bevacizumab and chemotherapy, specifically paclitaxel and carboplatin, in advanced nonsquamous NSCLC. As part of a larger phase III trial, this combination (PCBA) proved superior to the “standard” combination of bevacizumab/paclitaxel/carboplatin (PCB). In the wild-type population, the median PFS was 8.3 months compared with 6.8 months for the control arm, with a hazard ratio (HR) of 0.59 and further separation of the PFS curves beyond the median, presumably when patients had completed systemic chemotherapy and were on maintenance bevacizumab. This PFS benefit translated into an OS benefit in the intent-to-treat population: median OS of 19.8 months for PCBA vs 14.9 months for PCB with an HR of 0.76. Results were even more impressive in a subpopulation of TKI-exhausted patients with oncogenic drivers, either EGFR mutation or ALK translocation, where the HR for OS dropped to 0.54 (median OS not reached vs 17.5 months).
Although these results on their own are astounding, it remains to be seen if this regimen offers any therapeutic advantage over combination pemetrexed/carboplatin and pembrolizumab (PCP), which, in a similar population, resulted in an even more impressive PFS and OS benefit. The PCBA regimen is restricted to patients who are bevacizumab eligible, which would exclude many individuals with recent thromboembolic disease or recent history of hemoptysis. In addition, paclitaxel, in lieu of pemetrexed, is potentially more toxic with heightened risk of peripheral sensory neuropathy and alopecia. The addition of a fourth agent to a standard three-drug regimen can further exacerbate toxicity. Finally, the FDA approval explicitly omits the TKI-refractory population, which seemed to enjoy the greatest relative benefit in IMpower 150. Of note, this population was excluded from enrollment on the KEYNOTE-189 study, which led to pembrolizumab’s approval in combination with pemetrexed and carboplatin in advanced, nonsquamous NSCLC. In the absence of such an approval, the standard regimen in TKI-refractory patients remains elusive and controversial.
In this regard, we believe the FDA has missed the mark by failing to include the TKI-refractory population with oncogenic drivers in this important approval. ✦
Corey Langer, MD, ILCN Editor
Fabrice Barlesi, MD, PhD, ILCN Associate Editor
Caicun Zhou, MD, PhD, ILCN Associate Editor
Edgardo S. Santos Castillero, MD, FACP, IASLC Publications Committee Chair
New Approval for Atezolizumab
December 6, 2018 — The U.S. Food and Drug Administration (FDA) approved atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin for management of metastatic nonsquamous NSCLC with no EGFR or ALK mutations in the first-line setting. Atezolizumab is also approved by the FDA for treatment of patients with metastatic NSCLC who experience disease progression during or following treatment with platinum-based chemotherapy, as well as for those patients who have EGFR or ALK mutations and have experienced disease progression during or after targeted therapy.