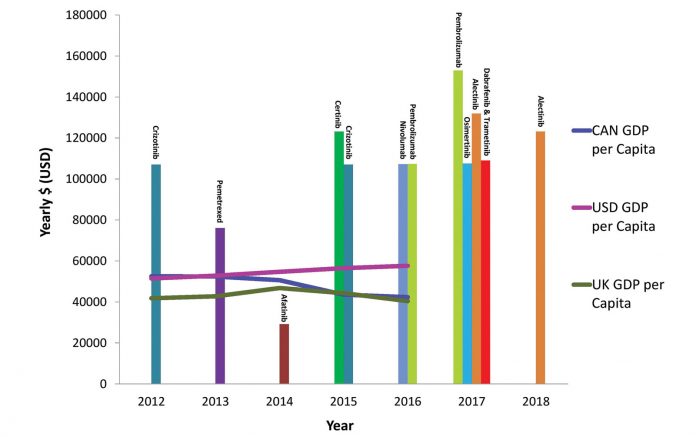
Abbreviations: GDP, gross domestic product; CAN, Canada; USD, United States; UK, United Kingdom.1,2
By Doreen A. Ezeife, MD, FRCPC; Malcolm Ryan; and Natasha B. Leighl, MD, MMSc, FRCPC, FASCO
Posted: March 1, 2019
Dramatic improvements in lung cancer outcomes can be attributed to recent advances in novel targeted therapy and immunotherapy. As the treatment paradigm for lung cancer evolves, concerns about the rising cost of these novel therapeutics has become a global dilemma. In Canada, the average price of lung cancer drugs increased by 17% between 2012 and 2017, whereas the gross domestic product per capita actually decreased by 19% during this time period (Fig. 1).1,2 Classic market forces do not consistently apply to cancer drug pricing, with higher prices paid for treatments of lower value, and next-in-class agents often costing as much or more than first-in-class agents.3,4

The Effects of Cost
Payers around the world are struggling to provide timely access to breakthrough lung cancer therapies for our patients. Uptake of novel cancer therapies is limited by high drug acquisition and technology or testing costs. For example, in the Canadian universal publicly funded healthcare system, only nine out of 14 recently approved lung cancer drugs have received public funding due to budgetary constraints, often after significant delays.1,5 In other high-income nations, such as Spain and Japan, fewer than half of all newly approved drugs are funded and available to all patients.6 The proportion of available novel anticancer therapeutics drops dramatically in lowand middle-income countries (LMICs). Although prices may be subsidized or reduced in LMICs, recently developed treatments remain unobtainable for most patients with lung cancer in these areas. In countries with private or hybrid healthcare systems, patients experience the negative consequences of expensive cancer treatments directly. In the United States, for example, high out-of-pocket payments are a reality for many patients with advanced lung cancer and can affect adherence to therapy, potentially leading to treatment failure and drug resistance. A recent U.S. study found that patients with lung cancer receiving tyrosine kinase inhibitors (TKIs) were more compliant with therapy if their co-payments were less than $30 USD monthly, compared to those with higher co-payments.6 Previous studies have shown that patients diagnosed with lung cancer in the United States are almost four times more likely to declare bankruptcy, and that bankruptcy is associated with higher cancer mortality.7 Other research in the United States has shown that patients with lung cancer with less financial reserve experience worse quality of life and higher symptom burden.8

Costs and Decision Making
To make informed policy decisions about costly advances in cancer care, a growing number of countries such as Canada and the United Kingdom have expanded the evaluation of novel therapies beyond clinical benefit and safety to include the economic effects on the healthcare system and the individual. In Canada, the patient perspective and system effect related to adoption of a new therapy, as well as the clinical benefit and economic effect, are all considered in decision making, as part of the Pan-Canadian Oncology Drug Review process.1 Cost-effectiveness evaluation helps us understand how treatment benefit relates to cost and can be expressed using the incremental cost effectiveness ratio (ICER)—the incremental cost of a novel therapy over the incremental benefit compared to the current standard of care. Thus, a treatment that significantly prolongs survival with low cost would be highly cost effective with a low ICER, compared to a treatment that only minimally affects survival or quality of life and yet is expensive, leading to a high ICER. However, there is no consensus on what constitutes an acceptable ICER threshold for funding a novel therapy, with significant variation across countries and disease types. The National Institute for Health and Care Excellence in the United Kingdom has a threshold of up to £30,000 per quality-adjusted life year (QALY) to be considered cost effective for National Health Service funding,9 although drugs with higher ICERs have been recommended for access through their Cancer Drugs Fund. In North America, the $100,000 per QALY threshold has been cited for oncology,10 although many drugs exceed these thresholds, often running over $220,000/QALY.11,12

Moving Forward
Many countries are increasingly using managed-entry agreements, which allow cost sharing between the payer (or government) and drug manufacturer when there is uncertainty regarding benefit and cost effectiveness. These programs strive to ensure patient access to new drugs; however, pricing in managed-entry agreements is often confidential, resulting in limited transparency. Temporary access measures to bridge delays in funding treatment include clinical trials and compassionate access programs. However, the conduct of trials of next-in-class agents using outdated comparators raises ethical issues, as these are conducted in countries that cannot afford the current standard. Furthermore, access to compassionate programs is not always equal, with variable access between academic versus community centers and outlying versus urban areas.13
As we negotiate accessible drug prices, we must bring drug manufacturers, payers, clinicians, and patients into the discussion, across geographic areas, so as to ensure access and still encourage research. We must also investigate other avenues for promoting sustainability, both by containing costs and prioritizing value. Following evidence-based practice, choosing investigations and therapies wisely, and reducing the costs of drug development may all be opportunities to contain current costs. Maintaining a higher bar to adopt a new standard therapy— such as targeting a survival hazard ratio of less than 0.8 in advanced lung cancer trials—is also important. Selecting those patients most likely to benefit through biomarker development is also key; this requires ongoing research and translation to practice. Inflation of costs through institutional, clinical, and pharmacy markups must also be addressed, as well as the development of biosimilars, leveraging patent laws to encourage more favorable pricing, and re-evaluating whether competitive development of “me-too” agents by multiple companies is a wise investment of resources. From a societal perspective, our focus must also be on the prevention and cure of this disease, which will alleviate the burden of suffering and cost to future generations of patients. ✦
About the Authors: Dr. Ezeife is with the Tom Baker Cancer Centre, Calgary, Alberta, Canada, and is assistant professor at the Department of Medicine, University of Calgary, Canada. Malcolm Ryan is a student at McGill University. Dr. Leighl is with the Cancer Clinical Research Unit, Princess Margaret Cancer Centre, Toronto, Canada, and is associate professor, Department of Medicine, University of Toronto, Canada.
References:
1. pan-Canadian Oncology Drug Review. Canadian Agency for Drugs and Technologies in Health website. cadth.ca. Accessed June 20, 2018.
2. The World Bank. worldbank.org. Accessed June 20, 2018.
3. Del Paggio JC, Sullivan R, Schrag D, et al. Delivery of meaningful cancer care: a retrospective cohort study assessing cost and benefit with the ASCO and ESMO frameworks. Lancet Oncol. 2017;18(7):887-894.
4. Mailankody S, Prasad V. Five Years of Cancer Drug Approvals: Innovation, Efficacy, and Costs. JAMA Oncol. 2015;1(4):539-540.
5. Ezeife DA, Truong TH, Heng DY, Bourque S, Welch SA, Tang PA. Comparison of oncology drug approval between Health Canada and the US Food and Drug Administration. Cancer. 2015;121(10):1688-1693.
6. Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381-390.
7. Ramsey SD, Bansal A, Fedorenko CR, et al. Financial Insolvency as a Risk Factor for Early Mortality Among Patients With Cancer. J Clin Oncol. 2016;34(9):980-986.
8. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of Financial Strain With Symptom Burden and Quality of Life for Patients With Lung or Colorectal Cancer. J Clin Oncol. 2016;34(15):1732-1740.
9. McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733-744.
10. Albaba H, Lim C, Leighl NB. Economic Considerations in the Use of Novel Targeted Therapies for Lung Cancer: Review of Current Literature. Pharmacoeconomics. 2017;35:1195- 1209.
11. Djalalov S, Beca J, Hoch JS, et al. Cost effectiveness of EML4-ALK fusion testing and first-line crizotinib treatment for patients with advanced ALK-positive non-small-cell lung cancer. J Clin Oncol. 2014;32(10):1012-1019.
12. Aguiar PN Jr, Haaland B, Park W, San Tan P, Del Giglio A, de Lima Lopes G Jr. Cost-effectiveness of Osimertinib in the First-Line Treatment of Patients With EGFR-Mutated Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018 May 31. [Epub ahead of print].
13. Azad A, Franco M. Co-funded expanded access programmes for new oncology drugs: creating a two-tier system for Australian cancer patients? Intern Med J. 2013;43(7):843-844.