By Stephen V. Liu, MD
Posted: March 1, 2019

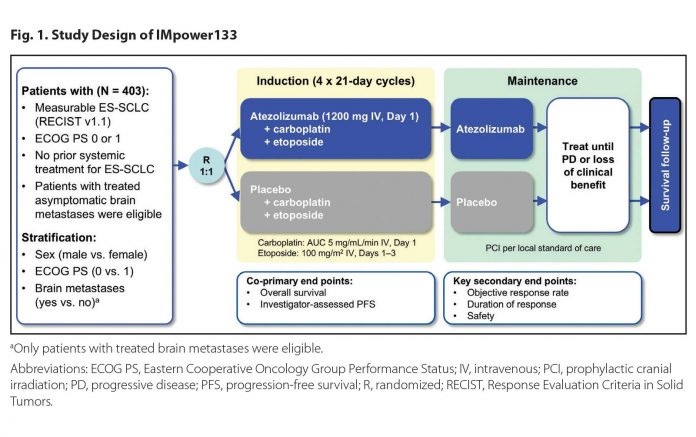
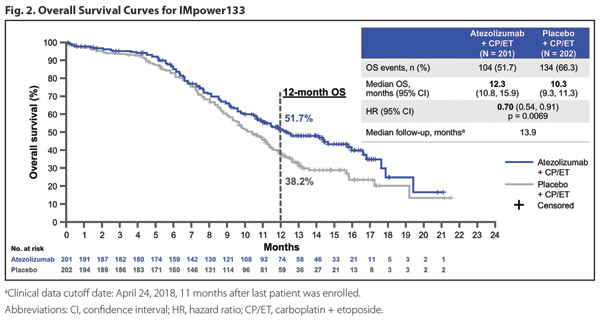
The addition of atezolizumab to standard chemotherapy for extensive-stage SCLC achieved what, for decades, had been beyond reach: an improvement in survival in this disease. Since the 1980s, our standard of care for SCLC has been a platinum chemotherapy agent plus etoposide. This regimen reliably provides an initial response that is often dramatic but is almost always short lived. IMpower133 was a global, randomized, double-blind, placebo-controlled trial designed to assess the effects of adding concurrent (and maintenance) atezolizumab to first-line carboplatin and etoposide in extensivestage SCLC (Fig. 1). As presented at the IASLC 2018 World Conference on Lung Cancer, the study met both coprimary endpoints with a significant improvement in overall survival (OS) and progressio-free survival (PFS). Median OS was 12.3 months with atezolizumab versus 10.3 months in the control arm (hazard ratio [HR] for death 0.70 [0.54, 0.91]; Fig. 2).1,2 The combination of atezolizumab, carboplatin, and etoposide was given a Category 1, preferred treatment recommendation in the National Comprehensive Cancer Network guidelines and awaits formal U.S. Food and Drug Administration approval.
Clinical Impact of IMpower133
In the context of more than 40 failed phase III trials, the improvement in survival seen in IMpower133 represents a tremendous achievement, and I have adopted this regimen as my new standard of care. PD-L1 expression was not used as a selection criterion, and blood–tumor mutational burden was not predictive of outcome. Although the search for a predictive biomarker continues, my current use of atezolizumab is for “all-comers” with extensive-stage SCLC.
There are limitations to the data, including patients with asymptomatic untreated brain metastases. Use of atezolizumab in this setting has not been explored; these patients were excluded from enrollment on IMpower133. No dataset perfectly encapsulates our daily clinic experience; we must extrapolate from available data. Because I would not expect these patients to derive less benefit from atezolizumab, I am comfortable with its use here, acknowledging the need for more data. IMpower133 also excluded patients with active autoimmune diseases, including paraneoplastic syndromes associated with SCLC. Here, I am very cautious, because worsening of these syndromes can be unsafe and can negatively affect quality of life.
How would I approach a patient who begins chemotherapy during hospitalization, where atezolizumab may not be available? I would likely add atezolizumab to the next possible cycle. Whenever feasible, however, I favor concurrent administration with the first cycle, to capitalize on the potential synergy between chemotherapy and atezolizumab. SCLC can have an unpredictable course, and delaying implementation may result in a lost opportunity. SCLC is an unforgiving disease and, too often, our first attempt at treatment is our only chance to affect the natural history of this highly lethal malignancy.
The Start of a New Chapter?
IMpower133 is only the first of several studies expected to read out over the next 2 years in SCLC. KEYNOTE-604 (NCT03066778) will assess the effects of pembrolizumab with chemotherapy, and CASPIAN (NCT03043872) will report the efficacy of durvalumab with or without tremelimumab in combination with chemotherapy. In addition, CheckMate 451 (NCT02538666) examines a maintenance approach after chemotherapy with nivolumab, nivolumab plus ipilimumab, or placebo. This is a different patient population—one that has completed chemotherapy and is well enough to enroll on a clinical trial— but hopefully this will also prove to be an effective strategy. Many more questions remain, including the role of consolidative thoracic radiation. Although the CREST study, which explored this approach in patients who responded to initial chemotherapy, did not meet its primary endpoint of improving 1-year survival, it did show an improvement in 2-year survival.2 It is important to note that consolidative radiation was not permitted in IMpower133, and additional study is needed not just to demonstrate efficacy but also to ensure safety when combining definitive radiation with atezolizumab and chemotherapy. Still, the potential is alluring, given the survival gains seen for patients with locally advanced NSCLC with durvalumab after chemoradiation.3 Examining the role of checkpoint inhibition after chemoradiation for limited-stage SCLC is also of great interest for the same reason.
Lest we forget the recalcitrant nature of SCLC, two recent immunotherapy studies yielded disappointing results. CheckMate 331 compared second-line topotecan to nivolumab and, unfortunately, did not meet its primary endpoint of improving overall survival.4 In IFCT-1603, second-line atezolizumab monotherapy was associated with a disappointing PFS of 1.4 months, a 6-month PFS rate of 6.3%, and a response rate of only 2.3%.5 Rovalpituzumab tesirine, an antibody-drug conjugate targeting DLL3, was explored in the singlearm TRINITY study and resulted in a response rate (assessed by independent radiology committee) of only 12.4%, with a median survival of less than 6 months.6 These sobering results further underscore the need for additional study. With the survival gains seen in IMpower133, we have finally moved the needle in SCLC. It is now our charge to build upon these results and ensure that the next major advance is not another 30 years in the future. ✦
References:
1. Horn L, Mansfield AS, Szczesna A, et al. First line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018 Sep 25. [Epub ahead of print].
2. Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015; 385(9962):36-42.
3. Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018 Sep 25. [Epub ahead of print].
4. Bristol Meyers Squibb. Press release. Available at: https://news.bms.com/press-release/corporate-financial-news/bristol-myers-squibb-announcesphase-3-checkmate-331-study-doe. Accessed November 14, 2018.
5. Pujol J, Greillier L, Audigier Valette C, et al. A randomized non-comparative phase II study of anti-PD-L1 atezolizumab or chemotherapy as second-line therapy in patients with small cell lung cancer. Ann Oncol. 2018; 29(suppl_8):viii596-viii602.
6. Carbone DP, Morgensztern D, Le Moulec S, et al. Efficacy and safety of rovalpituzumab tesirine in patients with DLL3-expressing, ≥ 3rd line small cell lung cancer: Results from the phase 2 TRINITY study. J Clin Oncol. 2018; 36(15);8507.
About the Author: Dr. Liu is an Associate Professor of Medicine at the Lombardi Comprehensive Cancer Center of Georgetown University.