By Ross Soo, MB BS, PhD, FRACP
Posted: December 2018
The treatment of patients with advanced NSCLC harbouring an actionable oncogene with tyrosine kinase inhibitors (TKIs) directed at its oncogenic molecular alteration represents one of the most significant advances in lung cancer management. Another area of achievement in the treatment of advanced NSCLC has been the use of immune checkpoint inhibitors targeting programmed death receptor-1 (PD-1) and programmed death receptor ligand-1 (PD-L1). Although EGFR and ALK TKIs can induce rapid responses in a large number of patients with advanced oncogene-driven NSCLC, the duration of response is generally modest. In contrast, immune checkpoint inhibitors have the potential for durable disease control; however, the response rates are lower. Given this situation, research must address whether molecular targeted agents and immune checkpoint inhibitors can be combined safely to improve antitumor activity.
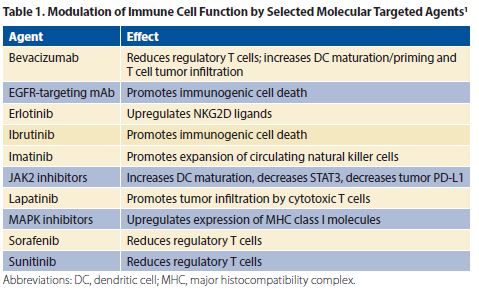
Immune cell function can be modulated by targeted therapy (Table 1).1 In pre-clinical studies, oncogenic EGFR and ALK signaling was reported to induce immune escape via the PD-1/PD-L1 axis.2,3 Some retrospective studies suggested an association between EGFR-positive or ALK-positive NSCLC and PD-L1 expression.3 However, other studies have reported an inverse relationship between PD-L1 expression and EGFR mutations and no association between PD-L1 expression and ALK rearrangement, as well as an uninflamed tumor microenvironment.5-7
Existing Data
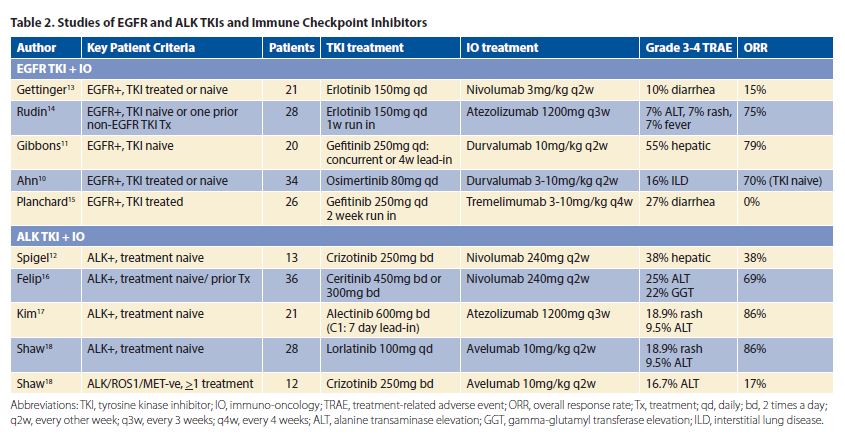
Early-phase studies of combination targeted therapy and immune checkpoint inhibitors in patients with pretreated or treatment-naive EGFR-positive or with EML4–ALK–positive NSCLC have been reported (Table 2). Response rates for combination EGFR TKIs or ALK TKIs plus immune checkpoint inhibitors are, at best, similar to previously reported response rates with EGFR TKIs/ALK TKIs alone, suggesting no synergistic effect. This observation has also been seen in preclinical studies of EGFR TKIs and immune checkpoint inhibitors8 as well as ALK TKIs and immune checkpoint inhibitors.9 Unfortunately, in some studies of combination EGFR/ALK TKIs plus immune checkpoint inhibitors, grade 3 to 4 treatment-related adverse events were unexpectedly higher than previously reported with TKIs or immune checkpoint inhibitors alone (Table 2).10-12 For example, in a phase I study of osimertinib and durvalumab (TATTON), an unexpectedly increased frequency of treatment-related adverse toxicity was reported, with 38% of patients developing interstitial lung disease (ILD) with grade 3 to 4 toxicity seen in 16% of cases. To place this in context, the incidence of ILD with single-agent osimertinib and durvalumab is 2.9% and 2%, respectively.10 Because of the high frequency of ILD observed, this treatment arm has been discontinued. In a phase I/II study of crizotinib and nivolumab, severe hepatic toxicities leading to the discontinuation of the combination treatment were seen in 38% of patients. In contrast, the discontinuation rates due to hepatic toxicities from single-agent crizotinib and nivolumab are about 2.3% and 0.3% to 1.5%, respectively.12
Current studies of combination molecular targeted therapy and immune checkpoint inhibitors in EGFR-positive or EML4-ALK–positive NSCLC are often associated with increased toxicities but not with greater activity; these combinations should not be administered in routine clinical practice. These findings highlight the importance of conducting well-designed phase I studies and also provide an opportunity to evaluate for biomarkers of efficacy and safety. Further studies on safety and activity of immune checkpoint inhibitors plus targeted agents are required in other oncogene-driven lung tumors such as KRAS, BRAF, or ROS1 NSCLC. Other research avenues being pursued include the characterization of the tumor microenvironment in oncogene-driven NSCLC, use of preclinical models and clinical studies for optimal treatment dosing, and sequencing and development of biomarkers to identify immunotherapeutic activity in early-phase trials. ✦
About the Author: Dr. Soo is a senior consultant, Department of Haematology-Oncology, National University Cancer Institute, Singapore, and an adjunct senior research fellow, Cancer Science Institute, National University of Singapore.
References:
1. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015;28(6):690-714.
2. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355-1363.
3. Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and down stream signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(17):4014-4021.
4. Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small cell lung cancer. Ann Oncol. 2014;25(10):1935-1940.
5. Soo RA, Lim SM, Syn NL, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer. 2018;115:12-20.
6. Dong ZY, Zhang JT, Liu SY, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145.
7. Toki MI, Mani N, Smithy JW, et al. Immune marker profiling and PD-L1 expression across Non-Small Cell Lung Cancer mutations. J Thorac Oncol. 2018 Sep 26. pii: S1556-0864(18)33124- 3311. [Epub ahead of print]
8. Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910-923.
9. Hong S, Chen N, Fang W, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2015;5(3):e1094598.
10. Ahn M-J, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol. 2016 Apr;11(4 Suppl):S115.
11. Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefi tinib (G): A phase I expansion in TKI-naive patients (pts) with EGFR mutant NSCLC. J Thorac Oncol. 2016;11(4 Suppl):S115.
12. Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation — Positive Advanced Non–Small Cell Lung Cancer (CheckMate 370). J Thorac Oncol. 2018 May;13(5):682-688.
13. Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol. 2018;13(9):1363-1372.
14. Rudin CM, Cervantes A, Dowlati A, et al. MA15.02 – Long-Term Safety and Clinical Activity Results from a Phase Ib Study of Erlotinib Plus Atezolizumab in Advanced NSCLC. Presented at: IASLC 19th World Conference on Lung Cancer; September 23-26, 2018; Toronto, Canada.
15. Planchard D, Barlesi F, Gomez-Roca C, et al. Phase I, safety, tolerability and preliminary efficacy study of tremelimumab (Trem) in combination with gefitinib (Gef) in EGFR-mutant (EGFR-mut) NSCLC (GEFTREM). Ann Oncol. 2016;27(suppl_6):1245P-1245P.
16. Felip E, De Braud FG, Maur M, et al. Ceritinib plus nivolumab (NIVO) in patients (pts) with anaplastic lymphoma kinase positive (ALK+) advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2017;35(15_suppl):2502.
17. Kim D-W, Gadgeel SM, Gettinger SN, et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). J Clin Oncol. 2018;36(15_suppl):9009.
18. Shaw AT, Lee S-H, Ramalingam SS, et al. Avelumab (anti–PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. J Clin Oncol. 2018;36(15_suppl):9008.