Posted: December 2018
In the following interview, Vassiliki A. Papadimitrakopoulou, MD, Jay and Lori Eisenberg Distinguished Professor of Medicine and section chief of Thoracic Medical Oncology at the University of Texas MD Anderson Cancer Center, discusses the findings and implications of IMpower132.
Q: Please explain the findings of IMpower132.
A: IMpower132 was a global, randomized, open-label, phase III study of 578 patients with stage IV nonsquamous NSCLC who were chemotherapy naive. Eligibility criteria included measurable disease by Response Evaluation Criteria in Solid Tumors guidelines v1.1 and Eastern Cooperative Oncology Group Performance Status 0-1. Exclusion criteria included tumors known to harbor EGFR driver mutations or ALK translocations, untreated central nervous system metastases, autoimmune disease, and prior exposure to immunotherapy. Patients were randomly assigned 1:1 to receive four or six cycles of carboplatin at a dose of AUC 6 mg/mL/min or to 75 mg/m2 of cisplatin plus 500 mg/m2 of pemetrexed every 3 weeks, followed by pemetrexed as maintenance therapy (Arm B) or to carboplatin/ pemetrexed or cisplatin/pemetrexed plus 1,200 mg of atezolizumab, followed by combination pemetrexed and atezolizumab as maintenance therapy (Arm A). Results of the study showed that the atezolizumab plus platinum pemetrexed–based chemotherapy (Arm A) resulted in improved progression-free survival (PFS; median 7.6 months versus 5.2 months for the control group) and was associated with a 40% reduction in risk for disease progression (hazard ratio [HR] 0.60, 95 CI [0.49, 072]) in all patients and across key clinical subgroups, including Asian patients (HR 0.42, 95% CI [0.28, 0.63]), never smokers (HR 0.49; 95% CI [0.28, 0.87]), and current and former smokers (HR 0.61, 95% CI [0.50, 0.74]). Also, at the interim overall survival (OS) analysis, atezolizumab plus pemetrexed-based chemotherapy demonstrated a numerical improvement in OS of 4.5 months over pemetrexed-based chemotherapy alone (HR: 0.81, 95% CI [0.64, 1.03]; p = 0.0797).
Q: Does IMpower132 change the therapeutic playing field in any way?
A: These IMpower132 study results are significant because they further support the use of atezolizumab plus chemotherapy in chemotherap-naive NSCLC. Changes in routine clinical practice might have to be considered after final reporting of the OS analysis in 2019. In addition, this study has the largest Asian subpopulation in a global checkpoint inhibitor combination trial in first line, treatment-naive NSCLC presented to date, with a remarkable HR of 0.42 in this group of patients. Therefore, further analysis of the significance of these findings is warranted. Additionally, the lack of increased benefit in patients with baseline liver metastases further supports the hypothesis that Socinski, Reck, and IMpower150 colleagues have presented, suggesting that the combination of bevacizumab and atezolizumab is required to confer clinical benefit in these patients.1
Q: Please explain the paradoxical results in those with lower levels of PD-L1 expression: The PFS confidence intervals crossed unity and there was no apparent survival benefit in this group, whereas benefits were more pronounced in those with higher levels of expression and in those with no expression. Why do you suppose the group of patients with no PD-L1 expression would have benefited more?
A: For this study, PD-L1 testing was not required, and approximately 60% of all patients had PD-L1 testing performed. Additionally, multiple factors could have affected these analyses including relatively small sample size, underperformance of the immunohistochemical assay, and potential enrichment of the PD-L1–low expression group with patients whose tumors harbored adverse genomic alterations (such as LKB1 loss). In addition, similar trends were previously seen in the analyses of KEYNOTE-0212 and IMpower131.3
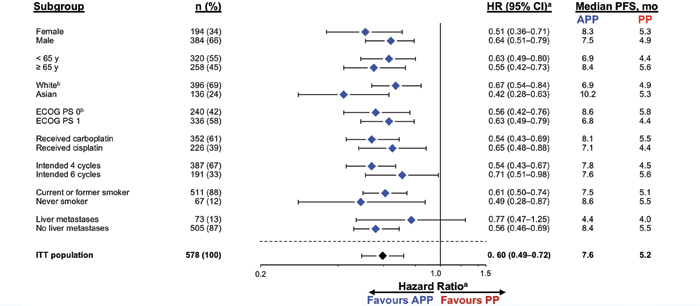
Abbreviations: APP, atezolizumab + carboplatin/cisplatin + pemetrexed; PP, carboplatin/cisplatin + pemetrexed. a Stratified HR for ITT; unstratified for all other subgroups. b Patients with other/unknown race (n = 46) and unknown baseline ECOG PS (n = 2) not included.
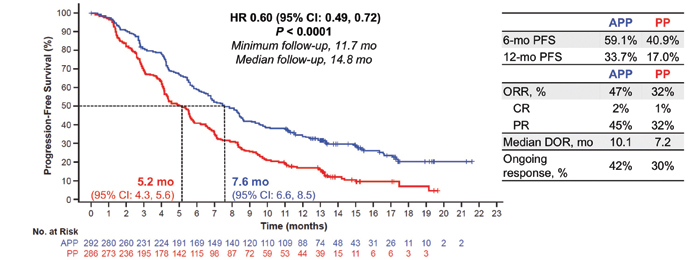
Abbreviations: APP, atezolizumab + carboplatin/cisplatin + pemetrexed; CR, complete response; DOR, duration of response; HR, hazard ratio; IRF, independent review facility; ORR, objective response rate; PP, carboplatin/cisplatin + pemetrexed; PR, partial response.
Q: Do you think the PD-1/PD-L1 inhibitors are largely interchangeable? Or does pembrolizumab (in light of the positive results of KEYNOTE-1894) hold some advantage over atezolizumab?
A: Cross-trial comparisons are not feasible because of differences in patient populations included, including distribution and prevalence of PD-L1 expression (in particular PD-L1 high), dose intensity of chemotherapy delivered, and differences in crossover to subsequent therapy. As such, definitive conclusions regarding the advantage of one checkpoint inhibitor versus another cannot be drawn. ✦
References:
1. Socinski MA, Jotte RM, Cappuzzo F, et al., for the IMpower150 Study Group. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378:2288-2301.
2. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497-1508. Presented at ESMO Immuno-Oncology Congress. Geneva, Switzerland: 2017.
3. Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. Presented at: American Society of Clinical Oncology Annual Meeting; Chicago, Illinois: June 4, 2018.
4. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078-2092.











