
By Kara Nyberg, PhD
Posted: October 2018
The Presidential Symposium at the IASLC World Conference on Lung Cancer 2018 (WCLC) showcased the top five scientific abstracts highlighting progress in detecting and treating different forms of lung cancer and mesothelioma. These presentations featured four positive clinical trials that advance current treatment standards and one negative clinical trial that reinforces the benefits achieved with the current standard of care.
CT Screening for Lung Cancer
Harry J. de Koning, MD, PhD, professor at Erasmus MC, Rotterdam, the Netherlands, presented the 10-year results of the NELSON trial, a randomized, controlled, population-based study evaluating the ability of low-dose CT screening to reduce the incidence of lung cancer mortality, as compared with no screening, in high-risk individuals.
The 15,792 participants in the trial were winnowed from a pool of more than 606,000 people aged 50 to 74 in the Netherlands and Belgium. This select group, who had smoked more than 15 cigarettes per day for more than 25 years or more than 10 cigarettes per day for more than 30 years, and who were still smoking or had quit 10 years ago or less, underwent random assignment to CT screening (conducted at baseline and 1, 3, and 5.5 years) or to no screening. Indeterminate nodules identified during screening were rescanned 2 months after initial detection to estimate nodule volume doubling time and to prompt a referral, if appropriate.
At 10 years after the start of the study, periodic CT screening reduced the rate of dying from lung cancer by 26% among high-risk men. Results were even more favorable for high-risk women, who benefitted from 61%, 53%, and 39% reductions in the rates of lung cancer mortality at 8, 9, and 10 years, respectively, after the start of random assignment.
Among the patients assigned to the screening arm, 86% complied with all four CT scans. Only 9.3% of participants required a follow-up CT scan within 2 months after a suspicious screening scan, which led to an overall referral rate of 2.2% and a lung cancer detection rate of 3.6%.
Based on these findings, Dr. de Koning concluded, “Volume CT lung cancer screening of high-risk former and current smokers results in low referral rates and a statistically significant reduction in lung cancer mortality in both genders.”
Durvalumab After Chemoradiotherapy in Stage III NSCLC
Results of the randomized, double-blind, placebo-controlled, phase III PACIFIC trial previously generated considerable excitement by demonstrating that use of the PD-L1 inhibitor durvalumab in patients with unresectable stage III NSCLC whose disease did not progress following platinum-based chemoradiotherapy— the standard of care—dramatically prolonged median progression-free survival (PFS) by almost 1 year when compared with placebo (16.8 vs 5.6 months; stratified HR, 0.52; 95% CI, 0.42- 0.65; p < 0.001). These findings marked the first major advance for such patients in many years and were sufficient to garner durvalumab approval by the U.S. Food and Drug Administration.
Scott J. Antonia, MD, PhD, of H. Lee Moffitt Cancer Center & Research Institute, presented updated findings from PACIFIC for overall survival (OS), the other primary endpoint, which proved equally impressive to the PFS results. Among the 713 randomly assigned patients (intent-to-treat population) who were followed for a median of 25.2 months, median OS has not yet been reached in the group that received durvalumab every 2 weeks for up to 1 year following chemoradiotherapy, whereas the median in the placebo group was 28.7 months (stratified HR, 0.68; 99.73% CI, 0.469-0.997; p = 0.00251; Fig.1). All patient subgroups, except those with <1% PD-L1 tumor expression, showed a survival benefit with durvalumab.
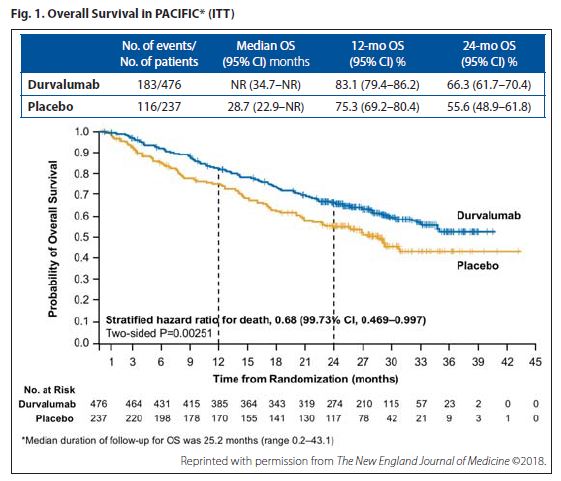
Results of the secondary endpoints bolstered the other primary endpoints. The time to distant metastasis or death (stratified HR, 0.53; 95% CI, 0.41-0.68) and updated incidence of new lesions (22.5% in the durvalumab group and 33.8% in the placebo group) heavily favored durvalumab over placebo.
Because PACIFIC is the first study to show a survival advantage following chemoradiotherapy in patients with unresectable stage III NSCLC, Dr. Antonia stated that use of durvalumab in the absence of disease progression constitutes the new standard of care for this patient population.
Brigatinib in Advanced ALK-Positive NSCLC
Brigatinib, a next-generation ALK inhibitor, stands poised to become a new first-line standard of care for patients with ALK-positive NSCLC—and one favored over crizotinib based on results emerging from ALTA-1L, a randomized, openlabel, phase III trial comparing brigatinib and crizotinib in 275 patients with stage IIIB/IV ALK-positive, ALK-inhibitor–naive NSCLC.
Brigatinib overcomes some of the shortcomings associated with crizotinib by readily penetrating the central nervous system (CNS) and remaining active against ALK mutations that render tumor cells resistant to crizotinib. Brigatinib has also consistently demonstrated the longest reported median PFS (16+ months) of any licensed or investigational ALK inhibitor in the post-crizotinib setting, so a head-to-head comparison of brigatinib and crizotinib as initial targeted therapy seemed inevitable.
D. Ross Camidge, MD, PhD, of University of Colorado Cancer Center, provided the first report from ALTA-1L, which was open to patients with asymptomatic brain metastases. Up to one prior systemic therapy regimen for locally advanced or metastatic disease was permitted, so long as it was not an ALK inhibitor.
Based on a median follow-up of only 9 to 11 months, the trial met the primary endpoint by demonstrating that brigatinib reduced the risk of progression or death by 51% compared with crizotinib, according to assessment by a blinded independent review committee (HR 0.49, 95% CI 0.33-0.74; p = 0.0007; Fig. 2). Although median PFS has not yet been reached for the brigatinib arm, 1-year PFS rates were 67% with brigatinib versus 43% with crizotinib.
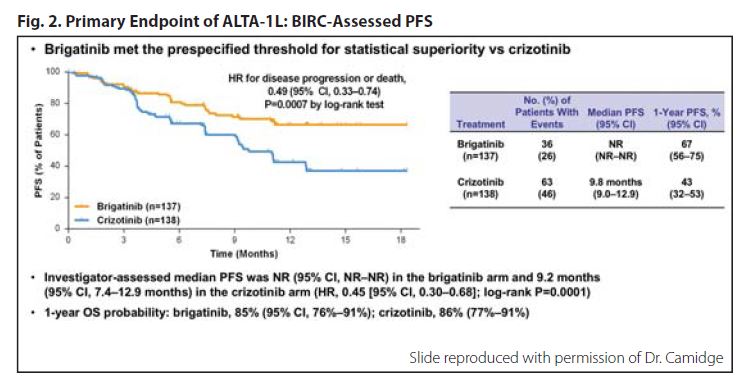
Brigatinib yielded more favorable PFS outcomes across all subgroups at this first interim analysis. However, the agent worked particularly well in patients with CNS disease at baseline. Among this subgroup of patients, the hazard ratio for PFS was 0.20 (95% CI, 0.09-0.46) in favor of brigatinib. Similarly, the hazard ratio for intracranial PFS was 0.27 (95% CI, 0.13-0.54).
Brigatinib appeared to be well tolerated. Although 29% of patients required dose reductions (vs 21% of patients treated with crizotinib), these were predominantly protocol-mandated for asymptomatic laboratory abnormalities (eg, increases in creatine phosphokinase, lipase, amylase). The ALTA-1L data suggest that early-onset pneumonitis may be a unique side effect of brigatinib compared with other ALK inhibitors, but this event occurred infrequently (3%).
Atezolizumab Plus Carboplatin and Etoposide in Extensive- Stage SCLC
The first-line standard of care for treatment of extensive-stage SCLC has long been a platinum-etoposide combination. However, IMpower133 appears poised to establish a new standard in the first-line setting—the fi st trial to do so in more than 30 years—that pairs a platinum doublet with immunotherapy.
Stephen V. Liu, MD, of Georgetown University, presented findings from this randomized, double-blind, placebo-controlled phase I/III trial that was conducted in 403 patients with untreated extensivestage SCLC, regardless of PD-L1 status. Participants received four 21-day cycles of carboplatin/etoposide combined with either atezolizumab or placebo as initial therapy, followed by maintenance therapy with atezolizumab or placebo until progressive disease or loss of clinical benefit.
IMpower133 met both co-primary endpoints at median follow-up of 13.9 months. Median OS reached 12.3 months in the atezolizumab arm versus 10.3 months in the placebo arm (HR 0.70, 95% CI 0.54-0.91; p = 0.0069), and median PFS reached 5.2 and 4.3 months, respectively (HR 0.77, 95% CI 0.62-0.96; p = 0.017). These benefits persisted across nearly all key patient subgroups evaluated, including all tumor mutation–burden subsets (ie, < 10, ≥ 10, < 16, and ≥ 16 mutations/ Mb; Fig. 3).
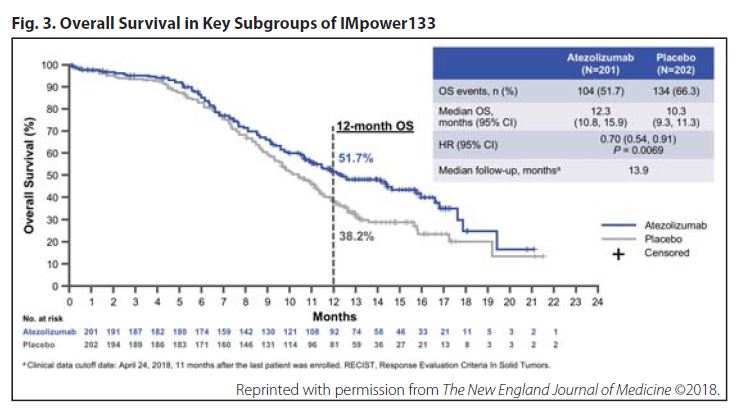
The addition of atezolizumab to chemotherapy resulted in a slight increase in toxicity. Whereas grade 3/4 adverse events (67.2% vs 63.8%%) and serious adverse events (37.4% vs 34.7%) were roughly similar between the atezolizumab and placebo arms, the greatest difference between the respective groups pertained to immune-related adverse events (39.9% vs 24.5%)—toxicities such as rash, hepatitis, and colitis, which occurred more frequently with atezolizumab, as expected. In addition, a greater proportion of patients assigned to the atezolizumab arm discontinued any study therapy compared with those in the placebo arm (11.1% vs 3.1%); withdrawal from atezolizumab was more prevalent than withdrawal from placebo (10.6% and 2.6%, respectively).
Nintedanib Plus Pemetrexed and Cisplatin in Unresectable MPM
Malignant pleural mesothelioma (MPM) is an uncommon cancer, but it is one increasing in frequency worldwide. Pemetrexed/cisplatin, the only approved regimen for the condition, provides a median OS duration of approximately 1 year. The randomized, double-blind, phase III LUME-Meso trial sought to improve upon this benchmark by adding the multikinase inhibitor nintedanib to pemetrexed/cisplatin.
Although initial signals from the phase II portion of the trial showed that the nintedanib-chemotherapy combination improved PFS over that of chemotherapy alone (9.4 vs 5.7 months; p = 0.017), with a trend toward improved OS, the phase III portion of the trial fell short in meeting the primary PFS endpoint.
As Giorgio V. Scagliotti, MD, PhD, of University of Turin and San Luigi Hospital, Torino, Italy, explained during his presentation of the LUME-Meso data, the trial has since been discontinued. Median PFS among the 458 patients with previously untreated, unresected epithelioid MPM included in the study reached 6.8 months for patients who received nintedanib plus pemetrexed/cisplatin and 7.0 months for patients who received placebo plus pemetrexed/cisplatin (HR 1.01, 95% CI, 0.79-1.30; p = 0.914; Fig. 4, online). An interim analysis of OS proved no more encouraging, with median values of 14.4 months and 16.1 months for nintedanib and placebo, respectively (HR, 1.12; 95% CI, 0.79-1.58; p = 0.538).
The safety profile of nintedanib was manageable and consistent with results observed in prior studies. Nintedanib did not compromise chemotherapy delivery or adversely affect patient quality of life.
Although disappointing that the addition of nintedanib to chemotherapy did not improve survival, the field can be reassured that pemetrexed/cisplatin continues to be the standard of care for unresectable MPM.









