By Edgardo S. Santos Castillero, MD, FACP
Posted: October 2018
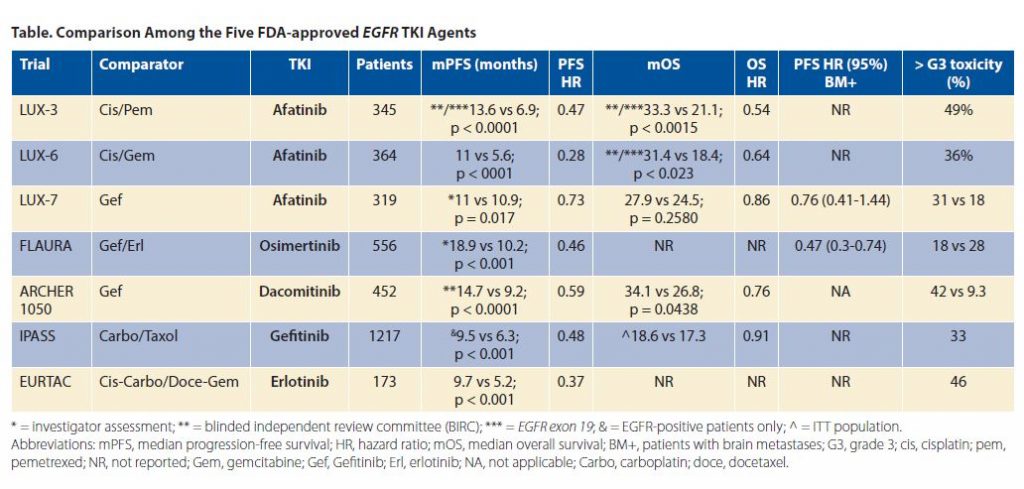
The approval of osimertinib in the frontline setting for patients with EGFR-sensitizing mutations has immediately challenged the decision-making process for oncologists. Osimertinib, a third-generation EGFR TKI, was initially developed as a specific therapy for EGFR T790M mutations, the most common mechanism of acquired resistance for first- and second-generation EGFR TKIs (i.e., gefitinib, erlotinib, afatinib, and dacomitinib). This mutation is present in 50% to 60% of patients at the time clinical resistance first manifests. In that scenario, osimertinib is the only available rescue drug for patients with this EGFR mutation at the time of first progression. On the other hand, when osimertinib is not used upfront, we preclude patients from receiving the EGFR TKI with the best progression-free survival (PFS) time to date (PFS 18.9 vs. standard TKI [gefitinib or erlotinib] 10.2 months, HR 0.46, 95% CI [037, 057]; p < 0.001).
However, T790M mutations are not a universal mechanism of resistance; hence, there is no guarantee that patients with refractory EGFR mutant tumors will be exposed to this compound. Based on the molecular evolution of these tumors, sequencing EGFR TKIs and reserving osimertinib for use at the time of documented resistance may make more sense if we want to generate the best long-term outcomes. Consequently, the dilemmas and controversies that arise from this topic deserve some consideration. When we treat a patient with EGFR mutations and advanced disease, we hope to induce a prolonged duration of response to a TKI, so therapy in this scenario is more like a marathon than a sprint. Certainly, multiple factors help determine first-line treatment decisions including available therapeutic options that vary tremendously from one part of the world to another, toxicity profile, existing comorbid conditions, presence of brain metastases, and cost.
Relevant Trials
Pooled overall survival (OS) data from AURA and AURA 2 studies showed a median OS of 26.8 months for patients who had been treated with osimertinib. Patients enrolled in those studies had been previously treated with a TKI and developed T790M at progression. The AURA 3 trial results, which showed superior PFS for osimertinib versus chemotherapy in the second-line setting after progression on an EGFR TKI due to emergence of a T790M mutation, resulted in the approval of osimertinib. Unfortunately, OS data are not yet available from the AURA 3 trial, which is critical as all those patients in second line received osimertinib (sequencing from first- to third-generation TKI).
Meanwhile, FLAURA demonstrated an unprecedented PFS time for osimertinib versus first-generation TKIs, including erlotinib and gefitinib. Here too, however, OS data are not yet mature, although early assessments look promising (p = 0.0068).
By the same token, ARCHER 1050, a phase III trial that compared dacomitinib (a second-generation EGFR TKI) to gefitinib in the first-line setting demonstrated superior PFS for dacomitinib, an agent that does not specifically target T790M mutations. This PFS was superior to those observed in other studies in the frontline setting, including IPASS (gefitinib vs. carboplatin/paclitaxel), EURTAC (erlotinib vs. physician’s choice chemotherapy), and LUX-Lung 3 and 6 (afatinib vs. cisplatin/pemetrexed or cisplatin/gemcitabine, respectively). ARCHER 1050 and LUX Lung 7 (a phase IIb randomized trial of afatinib vs. gefitinib) both prove that second-generation TKIs have more efficacy than gefitinib (category 1 by the National Comprehensive Cancer Network’s classification). Survival data from preplanned analyses of both LUXLung 3 and 6 clinical trials have been enumerated. For patients with EGFR exon 19 deletions OS was 33.3 months receiving afatinib in LUX-Lung 3 trial, compared to 21.1 months for chemotherapy (HR 0.54, 95% CI [0.36-0.79], p = 0·0015); in LUX-Lung 6, OS was 31.4 months for patients whose tumors harbored EGFR exon 19 deletions receiving afatinib compared to 18.4 months for chemotherapy (HR 0·64, 95% CI [0.44- 0.94], p = 0.023). Conversely, there were no differences in OS by treatment group for patients with EGFR exon 21 (L858R) mutations in either trial. Importantly, patients treated on LUX-Lung 3 did not receive second-line osimertinib at the time of disease progression, since it was not available at that time. Under these circumstances, one wonders if the use of a second-generation EGFR TKI, such as afatinib or dacomitinib followed by osimertinib or other agents, at the time of disease progression might be a preferable route. That is why the data presented at the recent annual meeting of the American Society of Clinical Oncology (ASCO) in June 2018 are relevant to this discussion.
Recent Data Presented at ASCO
ARCHER-1050 showed that dacomitinib improved OS over gefitinib (34.1 vs. 26.8 months, p = 0.0438; HR 0.76, 95% CI [0.582-0.993]). The nature of subsequent therapies given to these patients after dacomitinib progression (PFS1) is critical to this discussion. Of the 227 patients treated with dacomitinib, 27.8% subsequently received chemotherapy, 9.7% osimertinib, and 8.8% other TKI agents. Th ose patients exposed to osimertinib had an even better OS of 36.7 months. In September at the IASCL 19th World Conference on Lung Cancer, there were new data investigating the efficacy of dacomitinib at reduced doses because of its associated G3 toxicity (mainly dermatitis acneiform [13.7%], diarrhea [8.8%], paronychia [7.5%], and stomatitis [3.5%]). Dacomitinib was reduced from 45 mg daily to 30 mg (38.3% of the patients) or to 15 mg (27.8% of the patients) daily. PFS for those who received a reduced dose was 16.6 months vs 14.7 months for the ITT population. Interestingly, the group with the lowest dose (15 mg/day) had better PFS (31.2 months) than the groups receiving 30 mg/day (12.9 months) or 45 mg/day (9.1 months). Median OS has not yet been reached for the lowest-dose group, again demonstrating benefit over the other two doses (32.6 months for 30 mg/day and 22 months for 45 mg/day). As of the fall of 2018, it is not clear what the optimal EGFR treatment algorithm should be, with a median OS range between 33 to 37 months as the threshold.
To make matters more complicated, another landmark randomized phase II study (JO25567) presented by a Japanese group combined erlotinib with bevacizumab (75 patients) vs. erlotinib alone (77 patients) for treatment-naive patients with actionable EGFR mutations. In this randomized phase II study, survival follow-up results were presented. Erlotinib/bevacizumab showed superior PFS over erlotinib monotherapy (16.4 vs. 9.4 months, p = 0.005; HR 0.52, 95% CI [0.35-0.76]). Although this trial was not powered to assess the OS benefit of erlotinib/bevacizumab combination, the median OS was 47.0 for erlotinib/bevacizumab and 47.4 months for single agent-erlotinib. Moreover, OS for patients with EGFR exon 19 deletions was 53.2 and 50.9 months, respectively, for those receiving erlotinib/bevacizumab and erlotinib. In patients with EGFR exon 21 (L858R) mutations, OS was 43.6 vs. 42.1 months, respectively, for the combination arm and for erlotinib alone. The authors also reported post-discontinuation therapy up to third line, specifying the agents used. In the combination and erlotinib arms, 52% and 55% of the patients, respectively, were able to receive fourth or later lines of treatment; in this clinical scenario, 11 patients in each group received osimertinib. Moreover, we see encouraging OS data in both the intent-to-treat group as well as by mutation type.
In addition, a phase III study comparing erlotinib plus bevacizumab to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations (NEJ 026) was also presented at this year’s ASCO meeting. The primary endpoint of PFS was met; 16.9 vs. 13.3 months (HR 0.605, 95% CI, [0.417-0877]; p = 0.01573). OS, a secondary endpoint, was not mature. These two trials (JO25567 and NEJ 026) close the gap further between first- and third-generation TKIs in terms of PFS, which has been the endpoint for comparison among EGFR TKI agents until now. Specifically, the PFS results for combination erlotinib/bevacizumab in these two studies approaches that observed with osimertinib alone upfront but potentially preserves the option of administering osimertinib if T790M mutation emerges as a mechanism of resistance.
Deciphering the Data
This underscores the controversy. Should we treat all patients with EGFR exon 21 mutations or exon 19 deletions with osimertinib upfront, based on the PFS data of FLAURA as well as other factors such as improved central nervous system penetration (overall response rate 77% by blinded independent central review) and the lowest toxicity profile to date among all approved TKIs? Or should we treat patients with actionable EGFR mutations with second-generation TKIs and reserve osimertinib for the second line at progression (PFS1) if T790M emerges?
From my perspective, patients with an EGFR exon 21 mutation or exon 19 deletion with brain metastases at presentation should be considered for first-line osimertinib. For those patients with an exon 19 deletion (which is more sensitive to EGFR TKIs than exon 21 defects) and no known brain lesions, the decision for physicians is more difficult and likely requires an individualized discussion with each patient.
Moreover, how to handle disease progression on osimertinib is not very clear at this moment. Based on the FLAURA study, certain novel genomic abnormalities appear, but no standard effective treatments are available, especially for the C797S mutation and other variants in the same EGFR codon. As a result, most patients will need to proceed to chemotherapy alone or to the chemoimmunotherapy combination.
With this in mind, observations from the IMpower 150 trial, presented at the 2018 American Association for Cancer Research Annual Meeting, are relevant. This study, which compared the E4599 platform (carboplatin/paclitaxel/bevacizumab; triplet regimen) to the same platform plus atezolizumab (quadruplet regimen), included patients who had TKI-refractory EGFR-mutant (+) tumors. The results showed significant improvement in both PFS and OS for the entire population, and the presence of EGFR mutation did not seem to alter the results. In fact, that group fared better than the overall intent-to-treat population; the hazard ratio for PFS benefit in those with actionable EGFR mutations was 0.41 compared to 0.61 for the entire population. The OS for the intent-to-treat wild type group was 19.2 vs 14.7 months in favor of the quadruplet vs the triplet regimen (HR 0.78, 95% CI 0.64-0.96; p = 0.0164). The HR for OS in the EGFR/ALK (+) patients only was 0.54.
Consequently, from my perspective, it is perfectly permissible if a clinician decides to use a sequential approach, particularly because we still lack longterm OS data from the FLAURA and AURA 3 trials, both of which involved the use of osimertinib. Data from these two trials are eagerly awaited and will help to clarify the final picture. The OS data from ARCHER 1050 established a higher bar, especially now with updated data for clinical efficacy (PFS and OS) at lower doses if dose reduction is necessary to manage grade 2 or higher toxicity. A more cautious approach, rather than a wholesale switch to osimertinib, would be to wait for these studies to mature. If an EGFR sequencing approach is chosen by the oncologist, the patient must be closely monitored for progression of disease, and at the first manifestation of disease progression, all possible detection methods for T790M should be undertaken, including tissue re-biopsy and/or liquid biopsy. When using TKI sequencing, patients must understand that there is only a 50% to 60% chance that T790M will be documented at the time of disease progression, in which case the default option is systemic chemotherapy with or without immunotherapy. At the same token, if osimertinib is chosen at frontline, at the time of recurrence, the default option will be systemic chemotherapy or chemoimmunotherapy.
The extent to which osimertinib can increase the OS benchmark after afatinib failure is unknown; for dacomitinib, that figure is 36.7 months. At the very least,
afatinib followed by additional treatment at progression offers a 33-month median survival (at a minimum) for patients with exon 19 deletion.
The extent to which osimertinib can increase the OS benchmark after afatinib failure is unknown; for dacomitinib, that figure is 36.7 months
For the time being, both approaches seem acceptable, particularly while we await final OS data from FLAURA, AURA 3, and other studies. Based on data from the IMpower 150 trial, immunotherapy combined with chemotherapy and an antiangiogenic agent may result
in unprecedented OS rates and would constitute a very reasonable therapeutic option once a patient’s tumor becomes TKI refractory, regardless of whether osimertinib is used in the first or second line.
About the Author: Dr. Santos Castillero is the medical director of cancer research at the Lynn Cancer Institute in Boca Raton, Florida, clinical associate professor at Charles E. Schmidt College of Medicine Florida Atlantic University, and the present chair of the IASLC Publications Committee.











