By Shalini K. Vinod, MBBS, MD
Posted: October 2018
The standard of care for patients with inoperable stage III NSCLC is curative radiotherapy and concurrent chemotherapy.1 However, there are numerous factors that may preclude this approach, including poor respiratory function and large tumor volume—both of which are surrogates for unacceptable radiation doses to normal lung tissue resulting in high risk of pulmonary toxicity. Other more subjective factors include Eastern Cooperative Oncology Group performance status (ECOG PS), comorbidities, and age. In practice, 57% to 61% of patients with stage III NSCLC are treated with palliative radiotherapy.2,3 Patients not suitable for curative treatment usually receive single-modality palliative treatment given sequentially, with the order of treatment based on patient symptoms and disease burden.
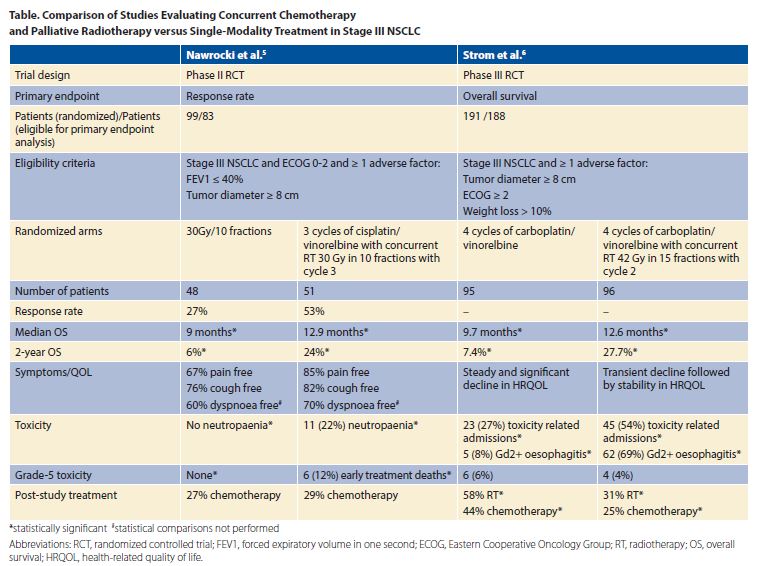
Until now, international guidelines have not specifically addressed palliative treatment of stage III NSCLC due to a paucity of evidence. The American Society for Radiation Oncology (ASTRO) has updated its palliative radiotherapy guideline to recommend palliative hypofractionated radiotherapy and concurrent chemotherapy for patients with stage III NSCLC who are deemed unsuitable for curative therapy.4 Patients must be fit for chemotherapy, have an ECOG PS of 0 to 2, and a life expectancy of at least 3 months. This guideline change is largely based on the publication of two randomized controlled trials.5,6 Both trials tested the efficacy of palliative hypofractionated radiotherapy and concurrent chemotherapy; however, the standard arms differed, with palliative radiotherapy featured in one5 and palliative chemotherapy in the other (Table).6
Supporting Data
Nawrocki et al. conducted a phase II trial of patients with stage III NSCLC unsuitable for curative treatment on the basis of FEV1 less than or equal to 40% and/or tumor diameter greater than 8 cm.5 Random assignment was to radiotherapy alone or two cycles of chemotherapy followed by concurrent radiotherapy. Patients receiving chemoradiotherapy had a significantly better median and 2-year survival and a similar rate of symptom relief compared to radiotherapy alone. Toxicity was greater in the chemoradiotherapy arm, with six early deaths (12%) versus 0 (0%) in the radiotherapy arm.
Strom et al. randomly assigned patients with stage III NSCLC unsuitable for curative treatment on the basis of one or more adverse prognostic factors (tumor size of 8 cm or greater, ECOG PS of 2 or greater, or weight loss of 10% or greater) to four cycles of chemotherapy or the same regimen with radiotherapy between cycles 2 and 3.6 Overall survival was significantly better with chemoradiotherapy (Table). Treatment-related mortality was similar; however, there were more hospital admissions and esophagitis in the chemoradiotherapy arm.
Neither study mandated PET staging, which could result in imbalances in otherwise unrecognized stage IV disease between arms. Differences in treatment on progression can also affect survival. Patients in the control arm of the study by Nawrocki et al.5 were less likely to receive palliative chemotherapy upon disease progression. Considering that these patients were chemo naive, one would have expected the majority of them to receive chemotherapy upon progression; however, this did not occur due to poor performance status. Interestingly, the converse was true in the study by Strøm et al.6, in which significantly more patients in the chemotherapy- alone arm received both further chemotherapy and radiotherapy. Despite some differences in eligibility criteria, the improvement in median and 2-year survival seen with concurrent palliative chemoradiotherapy in both studies was remarkably similar.
There is now evidence to support the use of concurrent chemotherapy and palliative radiotherapy in improving survival, symptoms, and quality of life in patients with stage III NSCLC who are unsuitable for curative treatment. –Shalini K. Vinod, MBBS, MD
Remaining Questions and Challenges
There is now evidence to support the use of concurrent chemotherapy and palliative radiotherapy in improving survival, symptoms, and quality of life in patients with stage III NSCLC who are unsuitable for curative treatment. However, the challenge remains in identifying patients who would be eligible for this approach without denying them the possibility of curative chemoradiotherapy. Curative radiotherapy in stage III NSCLC is underutilized, and this guideline should not be used as an excuse to treat patients palliatively. Tumor size alone should not be used as an indication for palliative treatment7 unless a safe radiotherapy plan respecting normal tissue tolerances cannot be generated. Similarly, it may be safe to treat patients with poor pulmonary function if the tumor volume, hence radiotherapy field is small. Performance status is a clearer indication of the ability to tolerate curative treatment; however, the survival benefit seen in Strom et al. was not seen in the subgroup with an ECOG performance status of 2 or greater. Significant weight loss is associated with poor prognosis and is often a marker of systemic disease, which would not have necessarily been detected in these studies in the absence of PET staging.
For patients with stage III NSCLC who are deemed unsuitable for curative treatment, concurrent chemotherapy and palliative radiotherapy is superior to either single modality alone. However, the optimal chemotherapy agents, radiotherapy doses, and scheduling are yet to be determined. Given the uncertainties in selecting patients for this treatment strategy, decisions are best made in the setting of a multidisciplinary team. ✦
About the Author: Professor Vinod is a radiation oncologist at Liverpool Hospital, Sydney, Australia, and a conjoint professor at the University of New South Wales.
References:
1. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199.
2. Vinod SK, Wai E, Alexander C, Tyldesley S, Murray N. Stage III non-small-cell lung cancer: population-based patterns of treatment in British Columbia, Canada. J Thorac Oncol. 2012;7(7):1155-1163.
3. Vinod SK, Simonella L, Goldsbury D, Delaney GP, Armstrong B, O’Connell DL. Underutilization of radiotherapy for lung cancer in New South Wales, Australia. Cancer. 2010;116(3):686-694.
4. Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018; pii: S1879-8500(18)30069.
5. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent Chemotherapy and Short Course Radiotherapy in Patients with Stage IIIA to IIIB Non-small Cell Lung Cancer Not Eligible for Radical Treatment: Results of a Randomized Phase II Study. J Thorac Oncol. 2010;5(8):1255- 1262.
6. Strøm HH, Bremnes RM, Sundstrøm SH, Helbekkmo N, Fløtten O, Aasebø U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
7. Ball DL, Fisher RJ, Burmeister BH, et al. The complex relationship between lung tumor volume and survival in patients with non-small cell lung cancer treated by definitive radiotherapy: A prospective, observational prognostic factor study of the Trans-Tasman Radiation Oncology Group (TROG 99.05). Radiother Oncol. 2013;106(3):305-311.











