Highlights from the IASLC 18th Lung Cancer Targeted Therapies Meeting keynote address.
By Suresh S. Ramalingam, MD
Posted: June 2018
EGFR-targeted therapy has entered a new phase as the result of several important advances in recent years. It is now well established that EGFR tyrosine kinase inhibitors (TKIs) are the preferred standard of care for patients with exon 19 or 21 EGFR mutations. TKI therapy provides superior response rates and progression-free survival (PFS) over platinum-based chemotherapy. This finding was followed by studies that described T790M as the mechanism of acquired resistance to EGFR TKIs in 50% to 60% of patients, which led to the development of osimertinib, a third-generation EGFR TKI that inhibits T790M as well as the common activating mutations.1
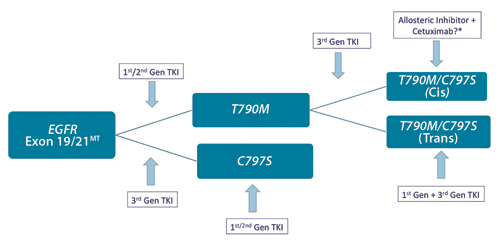
Figure. C797S-mediated Resistance: Clinical Implications
Osimertinib was proven to be superior to platinum-based chemotherapy in the setting of T790M-mediated acquired resistance. More recently, osimertinib was shown to be superior to erlotinib/ gefitinib as first-line therapy for EGFR mutated NSCLC, with a new benchmark PFS of approximately 19 months. It was also associated with more favorable activity against brain metastasis, less skin toxicity, and lower treatment-related serious adverse events. Consequently, osimertinib has emerged as a standard first-line therapy option for patients with an EGFR mutation. The mechanisms of resistance to osimertinib are just now beginning to be understood (Fig.), and several novel osimertinib-based combination approaches are under investigation.
Availability of plasma cell–free DNA platforms has greatly enhanced the ability to detect resistance mechanisms and shed light on prognosis. Emerging data suggest that early clearance of the mutation in plasma with TKI therapy is associated with favorable outcomes2; to the contrary, persistence of mutations in the plasma despite therapy is associated with shorter PFS and a lower response rate. The latter group of patients may be candidates for novel combination approaches even before the emergence of molecular resistance. There is also increasing evidence that co-mutations are frequently present in patients with EGFR activation mutations and could affect outcomes with TKI therapy.3 Further investigations into the role of co-mutations and the development of innovative treatment options based on these observations will likely result in better patient outcomes.
Immune checkpoint inhibitors that are widely used for the treatment of NSCLC have yielded disappointing results for patients with an EGFR mutation. Mutated tumors generally have lower PD-L1 expression and lower mutation burden. Chemotherapy is the preferred treatment for patients with EGFR mutations after acquired resistance to targeted agents. Elucidating the factors that drive the lack of sensitivity to checkpoint inhibitors is a crucial issue, and novel combination approaches are urgently needed in this patient population.
Whereas exon 19 and 21 mutations are the most common EGFR mutations, accounting for nearly 85% of all EGFR mutations, options for patients with less-common mutations are increasing. Afatinib was recently approved for patients with three distinct, uncommon EGFR mutations (L861Q, G719X, and S768I). Poziotinib and TK-788 are promising agents for patients with exon 20 insertion mutations. Poziotinib has demonstrated promising early results with a high response rate in a small cohort of patients4; data on PFS and duration of response are awaited. Currently, there are no targeted options for this molecular subset of patients.
The role of EGFR TKIs in patients with early-stage NSCLC is under investigation in randomized clinical trials. It is hoped that these studies will demonstrate the ability to cure more patients with the use of EGFR-targeted therapies. ✦
About the Author: Dr. Ramalingam is a member of the IASLC Board of Directors. Dr. Ramalingam is Roberto C. Goizueta Distinguished Chair for Cancer Research at Emory University School of Medicine, and the deputy director at Winship Cancer Institute of Emory University.
References:
1. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013; 19(8):2240-2247.
2. Mok T, Wu YL, Lee JS, et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin Cancer Res. 2015;21(14):3196-3203.
3. Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49(12):1693-1704.
4. Robichaux JP, Elamin YY, Tan Z, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018 Apr 23. [Epub ahead of print].











