Posted: April 2018
In January 2018, the response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) working group—an international collaboration of cancer treatment, scientific, and imaging experts— published a new guideline on addressing central nervous system (CNS) metastases in clinical trial designs for systemic agents.1 IASLC Lung Cancer News caught up with the guidelines’ lead author D. Ross Camidge, MD, PhD, Joyce Zeff Chair in Lung Cancer Research and Director of Thoracic Oncology at the University of Colorado Cancer Center, for a question and answer interview.
Q: How did these guidelines come about?
A: The first RANO-BM guidelines focused on defining the extent of the problem— how poor we have been at designing trials to appropriately include or exclude those with CNS disease, and the best way to generate data on CNS efficacy within such trials. They also described the “two-compartment model,” which means that CNS and extra-CNS efficacy data should be captured and presented separately, which is going to be key moving forward. These new guidelines now represent a very “how-to-do-it” description for improving clinical trial designs regarding drug development in cancers at risk of CNS spread. Obviously, lung cancer is one of the most significant cancers in this regard.
Q: Please summarize the three clinical scenarios for clinical trial design that you describe in the guideline.
A: At the start of the drug-development process you either suspect, based on previous clinical or preclinical data, that your drug is very unlikely to have activity in the brain (Scenario A), is very likely to have activity in the brain (Scenario B), or you just don’t know (Scenario C). In Scenario A, it’s all about protecting the patient and the drug-development process, but without inappropriately restricting access to a drug with activity in the rest of the body. So in Scenario A, the guidelines push to allow patients with CNS disease into a trial if the CNS disease is treated, asymptomatic, and stable, with each of these keywords being very clearly defined. In Scenario B, it’s about capturing CNS data accurately. So, for example, pursuing CNS imaging at the same frequency as body imaging, clearly defining what can and cannot be considered a CNS target lesion, and pushing for the two-compartment model in terms of data presentation. If a CNS signal is present, then we also propose “step-up inclusion criteria,” expanding eligibility to include symptomatic parenchymal disease and even leptomeningeal disease as the trial proceeds. In Scenario C, it’s about having a trial generate the data on CNS efficacy to then inform later Scenario A– or B– type designs as early as possible. So, for example, we propose adding dedicated CNS substudies at the recommended phase II dose in phase I trials, or rapid parallel CNS cohorts in later-phase trials.
 Q: What about how CNS data should be presented?
Q: What about how CNS data should be presented?
A: Looking back over the past few years, especially in thoracic cancers, it is astonishing how uncritical we have been about the supposed CNS efficacy data we have been presented with. The guidelines discuss in detail how we could do better. For example, why is it assumed that comparable progression-free survival in those with and without brain metastases tells us about a drug’s CNS efficacy if prior local treatment, which local treatment (whole brain versus stereotactic radiosurgery), and the number of CNS lesions are not described?
Q: What excites you about these new approaches?
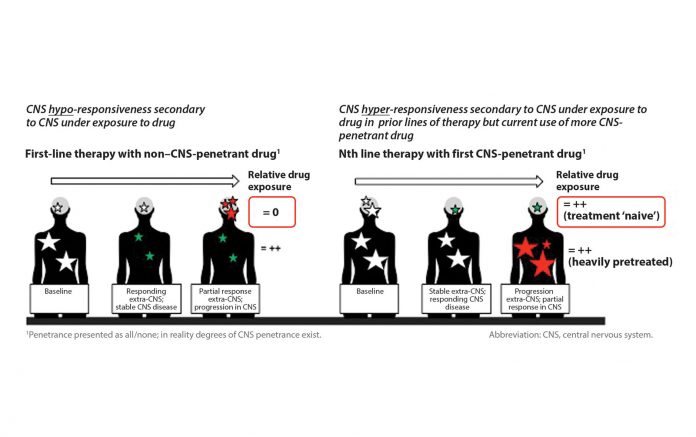
A: Recently, in later-line trials of some targeted agents, CNS lesions exposed to a highly CNS-penetrant drug are appearing hyper-responsive, with the CNS response rate seeming to exceed the extra-CNS rate, perhaps because the CNS lesions are behaving like disease in an earlier line of therapy. Whereas, for a less CNS penetrant drug, CNS lesions may appear hypo-responsive compared to the rest of the body (Fig. 1). Consequently, the overall response rate could be manipulated depending on the proportion of CNS versus extra-CNS disease included among the assessed target lesions. I think watching the field’s evolution regarding the demand for better-quality data that include the proportion of CNS disease contained within the overall target lesion dataset and present CNS and extra-CNS efficacy data separately will be amazing to see.
Q: Are we heading toward separate CNS drug doses and schedules being described?
A: For some drugs, yes. One of the other novel things in these guidelines is a description of how to formally explore dedicated CNS doses and schedules during drug development. So, in the absence of toxicity, dose and schedule exploration needn’t stop because a systemic target exposure or response rate was not seen. Instead, this exploration could continue among those with measurable CNS disease using the CNS response rate to see if higher continuous or intermittent doses are more beneficial for the CNS.
Q: Any final thoughts?
A: Just this: Over the next few years, our shared desire to improve outcomes should make us adopt clinical trial designs that more robustly address our clinical and research needs and engage in better presentations of relevant and accurately interpretable CNS data. If we do that, then everyone— patients, researchers, practitioners, and the pharmaceutical industry alike— will benefit. ✦
References:
1. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018 Jan;19(1):e20-e32. 2. Morgan RL, Camidge DR. Reviewing RECIST in the Era of Prolonged and Targeted Therapy. J Thorac Oncol. 2018 Feb; 13 (2):154–164.










