The U.S. Food and Drug Administration approved durvalumab on February 16, 2018, for the treatment of patients with stage III NSCLC whose tumors are unresectable and whose cancer has not progressed after treatment with chemoradiation— the first drug for this indication.
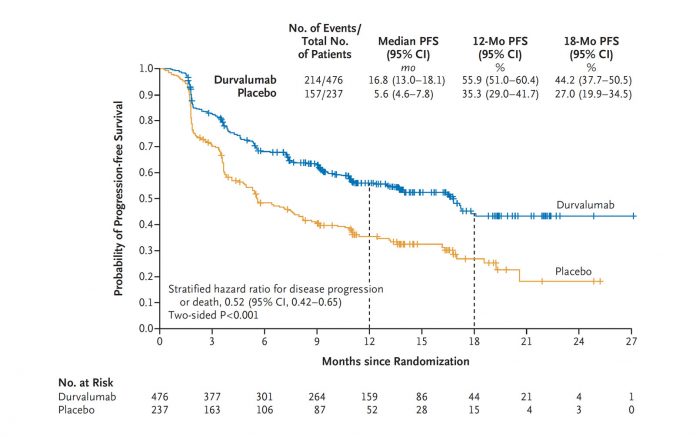
The approval was based on the phase III PACIFIC trial, a randomized trial of 713 patients in which sequential treatment with the PD-L1 inhibitor durvalumab was compared to placebo in patients with locally advanced, unresectable stage III NSCLC whose disease had not progressed following platinum-based chemotherapy concurrent with radiation therapy. The median progression-free survival (PFS) for durvalumab was 16.8 months compared to 5.6 months for placebo. Overall survival data have not yet been reported. ✦
|
Editor’s Note
Ostensibly, the PACIFIC trial is highly positive, with durvalumab yielding a striking, unprecedented PFS benefit compared to the control arm. However, some questions and concerns remain regarding this trial. Most concerning, a significant percentage of patients did not undergo pretreatment PET scanning, which has become standard in North America. Based on prior studies, approximately 15% to 20% of patients with apparent stage III NSCLC will be upstaged to stage IV by PET imaging; meaning, these patients were actually receiving durvalumab in the metastatic setting. Under these circumstances, durvalumab, we assume, would fare better than placebo. In addition, there was some imbalance in the percentage of patients with > 25% PD-L1 expression favoring the study drug. Also, there were no data on the percentage of patients who received < 60 Gy of radiation. Finally, the control arm appears to have underperformed historic controls—median PFS is usually 10 to 13 months. Obviously, overall survival data are needed to determine whether durvalumab truly is practice changing. We must see an increase in cure rates, not just in PFS, for routine use of durvalumab to be accepted, at least in the United States. However, cost may influence whether durvalumab is adopted worldwide. –Corey Langer, MD, Editor |