By Annemie Snoeckx, MD, and
Paul E. Van Schil, MD, PhD
Posted: April 2018
No universally accepted guidelines or management protocols exist for diagnosis and treatment of screen-detected nodules. Radiologic management of pulmonary nodules has made a substantial shift in the past decade. Research has shown that not all nodules are equal, and a tailored approach is required because of differences in lung cancer probability. Clinical setting, time of detection, and nodule characteristics determine which specific algorithm should be used.
To allow standardization and quality assurance in the management of screen-detected nodules, the American College of Radiology developed Lung-RADS, which is now the standard of practice for lung cancer screening in the United States. However, no European guidelines exist for screen-detected nodules. Although time of detection is not taken into account for incidental nodules, this differs for screen-detected nodules. Solid nodules detected during the baseline screening round are not equal to incident solid nodules (detected after baseline screening), and, in particular, those nodules that are new. The latter may be fast growing and have a higher probability of malignancy—with a cancer risk of 2% to 8%—thus requiring a different, more aggressive approach.1,2
Manual measurements are still the current practice in the daily evaluation of incidental nodules. It is, however, recommended that screen-detected solid nodules be assessed by semiautomatic volume measurements with calculation of volume-doubling time, as discussed in the IASLC Lung Cancer News February article about the recently published European position statement on lung cancer screening.1 Volumetric measurements are far more sensitive than two-dimensional measurements to detect growth.3 Size and growth are crucial criteria in the management of pulmonary nodules; nevertheless, careful morphologic evaluation (Figure 1) and use of lung cancer–prediction models may help in assessing malignancy risk.
Currently, nodule characterization with differentiation between solid and subsolid types determines management. However, this visual characterization suffers from a high degree of intra- and interobserver variability.4,5 Artificial intelligence and deep learning are reshaping the imaging world and may help solve this problem in the not-too-distant future. (See the article by Drs. Yanagawa and Tomiyama on page 8 for more on Deep Learning.)
Surgical Considerations
With the start of national and international screening studies, a new clinical problem has arisen for European thoracic surgeons— namely, how to deal with small pulmonary nodules and how to reduce the false positive rate. A recent paper by a task force of the European Society of Thoracic Surgeons (ESTS) addressed thoracic surgical issues6 and made 10 general recommendations concerning CT screening in Europe. Specific topics covered included implementation of CT screening in Europe, participation of thoracic surgeons in CT screening programs, training and clinical profile for surgeons, the use of minimally invasive thoracic surgery and related surgical issues such as quality control, and, finally, associated elements of CT screening (such as smoking cessation programs, radiologic interpretation, and pathology reports). This paper is highly recommended for European centers starting a screening program.
In general, it has been clearly demonstrated that the main goal of surgery for an invasive lung cancer is to obtain a complete resection, which, in and of itself, is a major prognostic factor. This mostly implies a lobectomy for tumors larger than 2 cm and at least a lobe specific systematic nodal dissection as defined in 2005 by a working group of the IASLC.7
The new adenocarcinoma classification published in 2011 by a common task force of the IASLC, American Thoracic Society, and European Respiratory Society and accumulating phase II data, mainly from Japan, has had important surgical implications. 8 New entities, including adenocarcinoma in situ and minimally invasive adenocarcinoma, were introduced. This new classification clearly resulted in a paradigm shift. The concept of sublobar resection, including wide wedge resection and anatomical segmentectomy, was reconsidered for smaller (< 2 cm), early-stage lung cancers. Anatomical segmentectomy is generally preferred to wide wedge resection because of concerns of local recurrence.9 Subcentimeter lung cancers—T1a disease—represent a specific subgroup, as they comprise the smallest lesions.10
Regarding the specific surgical approach, the ESTS task force recommends the use of minimally invasive procedures as much as possible.6 These are defined by using a camera and video monitor and access incision of less than 8 cm in length without any rib retraction, spreading, or resection. Video assisted and robotic-assisted procedures are also acceptable. For screen-detected cancers smaller than 3 cm, mortality should be less than 1%, morbidity less than 5%, and length of hospital stay approximately 3 days. ✦
About the Authors: Dr. Snoeckx is with the Department of Radiology, Antwerp University Hospital and Antwerp University, Belgium. Dr. Van Schil is with the Department of Thoracic and Vascular Surgery, Antwerp University Hospital and Antwerp University, Belgium.
Fig. 1. Chest CT in a 71-year-old Woman with a 50-Pack-Year Smoking History
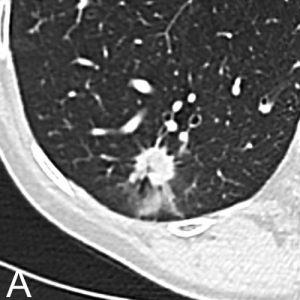
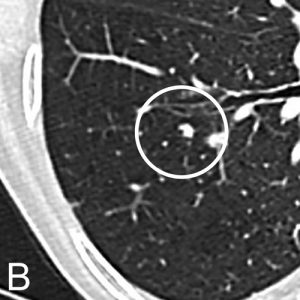
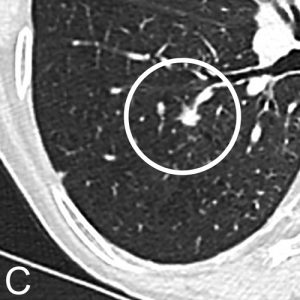
Chest CT in a 71-year-old woman with a 50-pack-year smoking history. Axial images in lung window setting show three nodules in the right lower lobe. Histopathologic examination after lobectomy showed that all three lesions had a different growth pattern, consistent with three primary tumors. The largest spiculated nodule with ground glass component (A) is an adenocarcinoma with predominant acinar growth pattern. The smallest lobulated nodule (B) is a moderately differentiated squamous cell carcinoma and the small spiculated nodule (C) is an adenocarcinoma with predominant lepidic growth pattern.
References
1. Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754-e766.
2. Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol. 2016;17(7):907-916.
3. Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax. 2017. Oct 22. [Epub ahead of print].
4. van Riel SJ, Sanchez CI, Bankier AA, et al. Observer Variability for Classification of Pulmonary Nodules on Low-Dose CT Images and Its Effect on Nodule Management. Radiology. 2015;277(3):863-871.
5. Ridge CA, Yildirim A, Boiselle PM, et al. Differentiating between Subsolid and Solid Pulmonary Nodules at CT: Interand Intraobserver Agreement between Experienced Thoracic Radiologists. Radiology. 2016;278(3):888-896.
6. Pedersen JH, Rzyman W, Veronesi G, et al. Recommendations from the European Society of Thoracic Surgeons (ESTS) regarding computed tomography screening for lung cancer in Europe. Eur J Cardiothorac Surg. 2017;51(3):411-420.
7. Rami-Porta R, Wittekind C, Goldstraw P, International Association for the Study of Lung Cancer Staging Committee. Complete resection in lung cancer surgery: proposed definition. Lung Cancer. 2005;49(1):25-33.
8. Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac Oncol. 2011;6(2):244-285.
9. Sihoe AD, Van Schil P. Non-small cell lung cancer: when to offer sublobar resection. Lung Cancer. 2014;86(2):115-120.
10. Van Schil PE. Non-small cell lung cancer: the new T1 categories. F1000Res. 2017;6:174.











